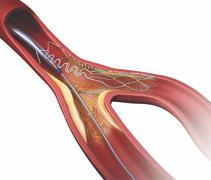
September 20, 2010 - Interim six-month results for a stent system designed to treat bifurcation lesions will be presented during the Transcatheter Cardiovascular Therapeutics (TCT) 2010 conference in Washington, D.C.
Tryton Medical will sponsor a symposium on Thusday, Sept. 23, to discuss the results of the Tryton side branch stent system study. David P. Foley, M.D., of the Beaumont Hospital & Royal College of Surgeons in Ireland, will go over the six-month clinical and angiographic results in a first-in-man study of the system, which is composed of more than 250 patients. Almost 200 patients in four different registries had a rate of target lesion revascularization of less than 4 percent.
The stent system has received CE mark approval in Europe and is commercially available in 21 countries throughout Europe and the Middle East. It is not approved in the United States.
“Current approaches to PCI of bifurcation lesions are diverse, and two-stent approaches in particular can increase the complexity and risk of acute complications and restenosis down the line,” Foley said. “The Tryton side branch stent offers a simple and straightforward approach to first securing the side branch, allowing stenting of the main vessel using a conventional drug-eluting stent. The Tryton solution has become an important part of my clinical practice, and I look forward to presenting additional data on the system at TCT.”
For more information: www.trytonmedical.com


 January 05, 2026
January 05, 2026 









