April 13, 2010 – More than 300 patients in Europe have been enrolled in a real-world study to assess the effectiveness of a coronary bifurcation stent. Enrollment was completed last week in E-Tryton 150 and E-Tryton Benelux, two European registry studies evaluating the Tryton Side Branch Stent System for the treatment of atherosclerotic lesions in the side branch at the site of a bifurcation.
“Early data for the Tryton Side Branch Stent System is very promising. The E-Tryton studies will help us understand the use of the Tryton Side Branch Stent System in real-world practice,” said Pieter R. Stella, M.D., Ph.D., director of the heart catheterization laboratories and clinical cardiovascular research at the University Medical Centre in Utrecht, the Netherlands, who participated in E-Tryton Benelux.
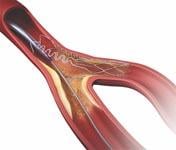
Areas of bifurcation in the vascular system are a common location for plaque and are particularly challenging to treat with currently available stent systems. As a result, the side branch is often left unstented, leaving it vulnerable to higher rates of restenosis. About 22 percent of patients treated for coronary artery disease have diseased bifurcated lesions.
The E-Tryton 150 study enrolled 151 patients, and E-Tryton Benelux enrolled 155. The primary endpoint of the studies is the overall rate of major adverse cardiac events (MACE) at six months following the procedure. MACE is defined as cardiac death, myocardial infarction and target lesion revascularization (main and/or side branch). The studies will also assess the technical success of the Tryton stent, procedural success, and the rate of target lesion revascularization (TLR) at six months after the procedure.
The Tryton Side Branch Stent is designed to offer a dedicated strategy for treating atherosclerotic lesions in the side branch at the site of a bifurcation. It uses a cobalt chromium stent that is deployed in the side branch artery using a standard single-wire balloon-expandable stent delivery system. A conventional drug-eluting stent is then placed in the main vessel.
The Tryton Side Branch Stent System demonstrated excellent six-month clinical results in a first-in-man study of the system in 30 patients, with no restenosis occurring in the side branch artery. The stent system has received CE mark approval in Europe, but is not approved in the United States.
For more information: www.trytonmedical.com


 January 05, 2026
January 05, 2026 









