June 30, 2020 — BioCardia, Inc. announced that the company has resumed cases in the CardiAMP Heart Failure Trial. The first patient procedure completed since elective procedures were paused due to COVID-19 took place at Morton Plant Hospital in Clearwater, Florida this month. New consents have also taken place at additional centers.
“We are pleased to be able to resume enrollment at several sites for our lead clinical program and look forward to cases taking place in July and August,” said BioCardia CEO Peter Altman, Ph.D. “As study centers resume elective procedures, we are working closely with our clinical partners to implement new FDA recommendations for clinical trials with respect to COVID-19 in order to minimize the impact on our patients, as well as the trial ahead. COVID-19 has been reported to cause heart damage, and some of our clinical investigators are seeing an increased amount of heart damage in patients who have avoided treatment due to fear of COVID-19 exposure. Our cardiac programs have the potential to help address this growing clinical need and are even more important in the current clinical landscape.”
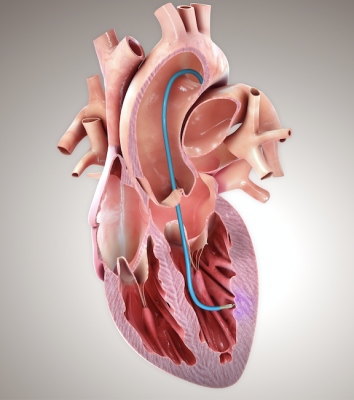
The CardiAMP Heart Failure Trial is studying CardiAMP cell therapy, an autologous bone marrow-derived mononuclear cell formulation designed to stimulate the body’s natural healing response in treating heart failure which develops after a heart attack. The trial is evaluating the cell therapy’s ability to improve patient survival, exercise capacity and quality of life, as well as its safety. The CardiAMP Heart Failure Trial is the first multicenter clinical trial of an autologous cell therapy to prospectively select patients based on cell potency to maximize the probability of patient benefit.
The ongoing multi-center, double-blinded, randomized (3:2), controlled pivotal CardiAMP Heart Failure Trial is expected to enroll 260 patients at up to 40 centers nationwide. The national co-principal investigators are Amish Raval, M.D., of the University of Wisconsin and Carl Pepine, M.D., of the University of Florida, Gainesville.
In March 2020, the Data Safety Monitoring Board indicated there were no safety concerns with the CardiAMP study results and recommended that the trial continue, as planned. To date, 75 patients have been enrolled at 25 active centers. The trial is sponsored, in part, by the Maryland Stem Cell Research Foundation and has reimbursement from the Centers for Medicare and Medicaid Services (CMS).
For more information: www.biocardia.com
Related CardiAMP Heart Failure Trial content:
How Biotechnology is Impacting the Treatment and Prevention of Heart Disease
CardiAMP Heart Failure Trial Design Presented at Texas Heart Institute Symposium
BioCardia Completes Roll-in Cohort in Pivotal Phase III CardiAMP Heart Failure Trial

 March 20, 2024
March 20, 2024