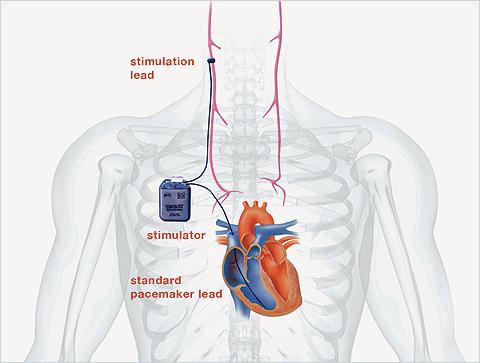
September 17, 2014 — BioControl Medical announced that it has reached an important clinical trial milestone, reaching 480 randomized subjects — or 70 percent — of the planned 650 subjects with congestive heart failure (HF) in the INOVATE-HF (INcrease Of VAgal TonE in Heart Failure) trial of its CardioFit system, the first medical device designed to treat HF using neurostimulation. With more than 65 participating centers in the United States and Europe, INOVATE-HF is the largest prospective, randomized global study ever to evaluate the treatment of HF with vagus nerve stimulation.
CardioFit is an implantable vagus nerve stimulation system that includes a pacemaker-like stimulator placed in the upper chest and a proprietary nerve stimulation cuff placed on the right vagus nerve in the neck. It is designed to help patients achieve optimal therapy parameters by providing stimulation only when a patient’s heart rate is between pre-set limits. The primary endpoint of the study is to compare the number of HF hospitalizations and all-cause mortality in patients with CardioFit vs. those on standard evidence-based management.
“Reaching the 70 percent milestone in the INOVATE-HF trial has been incredibly important to the entire company, as well as our investigators,” said Ehud Cohen, Ph.D., chief executive officer of BioControl Medical. “Since its inception, the entire team has been working tirelessly to move the study forward because we believe in the promise this pioneering technology holds for patients with heart failure.”
The milestone follows a recent grant of an additional U.S. patent on BioControl Medical’s technology, one of 45 that have been awarded over the past few years. The patent (No. 8,718,791) is for a nerve stimulation electrode that enables fiber selection within a nerve and is part of the company’s efforts to develop nerve simulation electrodes designed for specific clinical uses.
“No other nerve stimulation electrode on the market is specifically designed for cardiac application,” said Shai Ayal, Ph.D., chief technology officer, BioControl Medical. “This is an industry-leading technology and allows the CardioFit system to achieve stimulation currents of 3.5 millamperes and above, and activate the cervical vagus nerve in a way that no other system is capable. We believe this technology has made a tremendous difference in our INOVATE-HF trial.”
Initiated in April 2011, INOVATE-HF is an investigational device exemption (IDE) clinical study to determine the safety and efficacy of CardioFit in reducing hospitalization and death among patients with HF by comparing treatment with CardioFit to standard evidence-based management.[1] The study is randomized at a 3:2 ratio, so that for every five subjects enrolled, three are implanted with CardioFit and two are randomized to the control group. The company expects at least 80 percent of the patients in the trial will also have an implantable cardioverter defibrillator and 30 percent of patients will have a cardiac resynchronization therapy device.
More than 275 CardioFit implants have been completed to date as part of the INOVATE-HF study. Results of the study will be used to support a Premarket Approval Application to the U.S. Food and Drug Administration.
For more information: www.biocontrol-medical.com
References
1.Luscher, TF, et al. “The European Heart Journal and the European Journal of Heart Failure: partners in scientific publishing.” European Journal of Heart Failure (2012) 14, 1075–1082


 January 05, 2026
January 05, 2026 









