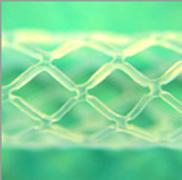
December 7, 2010 – A new bioresorbable stent scaffold can be overinflated by more than 25 percent without cracking or crazing. New data from Arterial Remodeling Technologies (ART) show that its bioresorbable polylactic acid (PLA) stent platform is designed to avoid malapposition.
With conventional stents a gap between the vessel and the stent can manifest. This "malapposition" of stent struts within the vessel has been shown to occur in conventional stent placements and is a significant risk factor in stent thrombosis. Additionally, data on other bioresorbable stents showed that when the struts have been overinflated to compensate for potential malapposition, they have cracked or crazed. This may lead to a life-threatening coronary event.
The company’s stent is designed to have a faster and smoother resorption; a non-crystalline polymer; better, homogeneous stress diffusion; and crack- and crazing-free expansion. In addition, the device is designed to be delivered by conventional stenting techniques, is balloon-expandable and meets the market standard of 6-French compatibility.
For more information: www.art-stent.com


 January 05, 2026
January 05, 2026 









