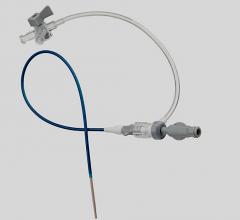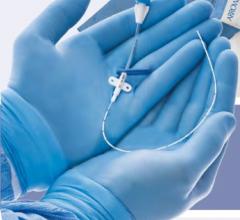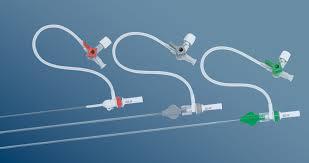
June 4, 2014 — Biotronik has expanded its line of reinforced introducer sheaths. Following up on the success of the 4 French compatible Fortress Introducer Sheath, the expansion maintains the flexibility and stability of the 4 French model, and is now available in 5 French and 6 French.
“Essential functions of a sheath introducer are to allow me the flexibility to maneuver through complex vascular anatomy, while maintaining stability to prevent any complications inserting and removing balloon catheters and stents,” said Flavio Airoldi, M.D., MultiMedica Hospital, Milan, Italy.
The Fortress Introducer Sheaths are reinforced with a resilient and flexible stainless steel coil, which provides a maximum degree of flexibility and stability to introduce a balloon catheter or self-expanding stent to treat diseased arteries. The coil simultaneously provides reinforcement to prevent kinks that can occur in the shaft during such procedures.
The 5 French and 6 French versions of the Fortress also retain a lubricious hydrophobic coating and smooth tapered tip design, making them easier to insert, and have been demonstrated to limit potential complications at the injection site.
The new Fortress series is now available in CE accepting countries.
For more information: www.biotronik.com

 June 10, 2020
June 10, 2020