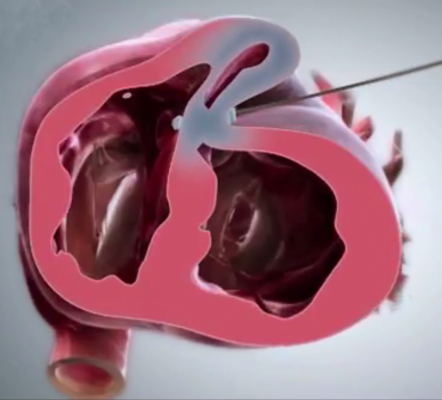
BioVentrix closed-chest Revivent-TC Ventricular Enhancement System reduces the size of the ventricle for more efficient pumping in heart failure patients.
December 29, 2015 — BioVentrix in mid-December announced the successful completion of the 30th clinical case using the closed-chest Revivent-TC Ventricular Enhancement System for heart failure (HF). The hybrid procedure was performed by Prof. Petr Neuzil (interventional cardiologist) and Dr. Ivo Skalsky (cardiothoracic surgeon) on a 38-year-old female patient at the Na Holmoce Hospital in Prague, Czech Republic.
The Less Invasive Ventricular Enhancement or the LIVE procedure, utilizing this new TransCatheter approach, is in clinical trials throughout Europe. This case comes on the heels of other successful Revivent-TC cases performed recently in Spain, Italy, France, and Germany.
"Before the LIVE procedure, this patient's quality of life was severely impacted by her heart failure to the point where she could no longer walk even short distances without discomfort. The Revivent-TC system allows treatment of patients with worsening heart failure and those that are already very ill without opening the chest," said Neuzil.
"The adoption of the Revivent-TC system allows us to treat the underlying cause of heart failure by reshaping the left ventricle to improve pumping efficiency and reduce wall stress," Skalsky added.
Recent analysis has shown that 76 percent of patients with mitral regurgitation that were treated with the Revivent technology had an added benefit of improvement in mitral valve functionality.
BioVentrix has reported an average reduction of 31 percent in Left Ventricular volume and a 14 percent improvement ejection fraction (EF) at one-year post-op follow-up. The data continues to show the same volume reduction and ejection fraction increase with this hybrid approach as was demonstrated with the previous surgical technique.
Placement of the Revivent-TC System via the LIVE procedure obviates the need for more invasive surgery. Instead, small titanium anchors are placed along the outer surface of the heart and along one of the interior walls via a closed-chest, TransCatheter approach. The anchors are then pulled toward one another, effectively excluding the scarred and non-functioning heart wall. Ventricular volume is immediately reduced as a result of the exclusion.
For more information: http://bioventrix.com


 November 14, 2025
November 14, 2025 









