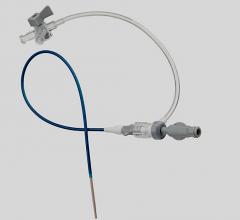
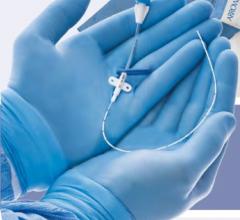
August 19, 2014 — Bluegrass Vascular Technologies closed $4.5 million in Series A financing, that will allow the company to obtain CE mark for the Surfacer Inside-Out Access Catheter System. Funds will also be used to enhance manufacturing capabilities, and proceed with U.S. regulatory submissions. The Surfacer System is a proprietary system that allows physicians to gain venous access using a novel inside-out approach.
"The Surfacer System addresses a significant unmet clinical need in the vascular access market by allowing physicians to gain access through a previously occluded vein," stated Alan Dean, senior managing director of Targeted Technology Fund II, and Bluegrass Vascular director. "With a growing market awareness of central venous occlusion and interest in maintaining access, we believe Bluegrass Vascular is well positioned to have a strong presence in the dialysis and chemotherapy markets and is a valued addition to our investment portfolio."
The Targeted Technology Fund is a venture capital firm focused on early stage healthcare companies that offer disruptive technologies with compelling market needs. A leader in funding medical technology innovation in the Southwest, Targeted Technology Fund II stated that Bluegrass Vascular will relocate their headquarters to San Antonio as part of the deal.
Approximately 6.5 million patients, worldwide, require central venous access (CVA) for medical treatment and it is estimated that more than 40 percent of those patients will develop a venous thrombosis, which may compromise their medical care. After all central veins become compromised, patients must resort to invasive surgical techniques to gain or maintain CVA. The Surfacer System maintains access in an occluded vein, halting the progression to invasive surgery and downstream health risks associated with poor circulation, using a less risky inside-out approach.
"This financing enables us to pursue CE mark applications and take us one step closer to providing a better solution for patients with venous obstruction," commented Jim Clifton, CEO of Bluegrass Vascular. "The Targeted Technology Funds have a strong track record of bringing ground-breaking medical technology to market. We are pleased they share our vision for the Surfacer System and we look forward to commercializing the product in Europe and United States."
For more information: www.bluegrassvascular.com

 June 10, 2020
June 10, 2020