January 24, 2018 — Boston Scientific Corp. announced it has closed an investment and entered into an acquisition option agreement with Millipede Inc., a privately-held company that has developed the IRIS Transcatheter Annuloplasty Ring System for the treatment of severe mitral regurgitation (MR). Under the terms of the agreements, Boston Scientific has purchased a portion of the outstanding shares of Millipede along with newly issued shares of the company for a total consideration of $90M. Boston Scientific has the option to acquire the remaining shares of the company at any time prior to the completion of a first in human clinical study that meets certain parameters. Upon the completion of the clinical study, Millipede has the option to compel Boston Scientific to acquire the remaining shares of the company. Each company's option period expires by the end of 2019. Completion of this acquisition would result in an additional $325M payment by Boston Scientific at closing with a further $125M becoming payable upon achievement of a commercial milestone.
MR is caused by a leaking mitral valve, which then causes blood to regurgitate from the left ventricle to the left atrium of the heart. Over time, the condition can lead to or accelerate heart failure and heart rhythm problems. Many patients with severe MR have compromised heart function and are not able to tolerate open-heart surgery to repair or replace the leaking valve. This large and currently underserved patient population could benefit from a fully-percutaneous transcatheter procedure that can repair the dilated mitral annulus and reduce regurgitation without undergoing surgery.
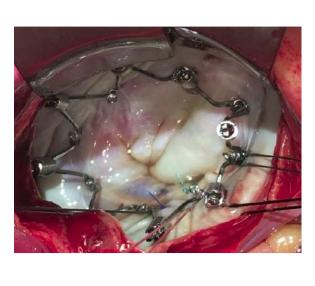
The Millipede IRIS annuloplasty ring, delivered via a transcatheter-transseptal delivery system, follows the standard surgical approach to repair and reduce the size of a dilated mitral annulus. The IRIS device is a complete ring designed to be used as a stand-alone device, or in combination with other technologies in patients with severe MR. The device is designed to be highly customizable to a specific patient's anatomy and disease state, and is repositionable and retrievable to promote a high-quality outcome.
Millipede is based in Santa Rosa, California. It was founded in 2012 by majority investor Santé Ventures and Steve Bolling, M.D., and has been led by CEO and Co-founder Randy Lashinski since 2014.
For more information: www.bostonscientific.com, www.millipedemedical.com
Related Mitral Valve Content
VIDEO: Update of Mitral Valve Repair and Replacement Technologies
VIDEO: Transcatheter Mitral Valve Implantation in Practice and Technologies in Development
Advances in Heart Valve Technology in 2017
Key Interventional Technology Trends at TCT 2017


 November 14, 2025
November 14, 2025 









