A live CoreValve TAVR case in the main arena at TCT 2017. Structural heart has moved from a science fiction discussion in future technology sessions at TCT a decade ago to now being a major portion of what the conference discusses for today's clinical practice.
Here is a list of what I think were some of the top interventional technologies discussed at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting this past fall. These technology areas have the biggest potential to impact clinical practice and received a lot of attention at this year’s meeting.
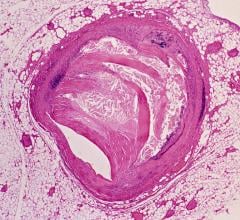
Bioresorbable Stents Suffer a Setback
The Abbott Absorb bioresorbable vascular scaffold (BVS) was the first commercialized bioabsorbable stent. Its U.S. Food and Drug Administration (FDA) approval in 2016 was by far the most popular breaking news item ever published by DAIC. However, all that enthusiasm went away in 2017 with new ABSORB clinical trial data showing poorer outcomes with Absorb over the current market-leading Xience stent. Abbott pulled the stent off the market worldwide last September due to slow sales and in anticipation of the release of new data showing a continued poor outcomes trend and that the device is difficult to use. Data from several ABSORB trials were among the top news makers at TCT 2017. Read the overview article “Current State of Bioresorbable Stent Technology.” I spoke with Ajay Kirtane, M.D., SM, director of the cardiac catheterization laboratories at New York-Presbyterian Hospital/Columbia University Medical Center, who has been involved in the ABSORB trials. Watch the interview in the video “What Went Wrong With the Absorb Stent?”
Mitral and Tricuspid Valve Interventions
Structural heart interventions encompass an ever-growing segment of TCT sessions. With FDA clearance for two devices for transcatheter aortic valve repair (TAVR) and the outstanding data that continues to flow from TAVR trials, a lot of attention has now turned to transcatheter repair or replacement in the mitral and tricuspid valves. This new technology area was the subject of seven late-breaking TCT device presentations. While the anatomy and issues involved with implantation in these valves are much more complex than the aortic valve, early clinical data appears very promising. I spoke with two of the top transcatheter mitral valve experts at TCT to find out the latest advances in this area. Watch the video interview with Ted Feldman, M.D., MSCAI FACC FESC, director of the cardiac cath lab, Evanston Hospital. Watch my interview with Adam Greenbaum, M.D., co-director, Center for Structural Heart Disease, Henry Ford Hospital. A key expert in the new area of transcatheter tricupid interventions is Rebecca Hahn, M.D., FASE, director of interventional echocardiography and professor of medicine at Columbia University Medical Center / New York-Presbyterian Hospital. Here is a link to a video when I spoke to her earlier in 2017 about tricuspid interventions.
Angiography Imaging-based FFR Assessments
Catheter-based fractional flow reserve (FFR) is branded as the gold standard for physiological assessment of coronary lesion severity, and to determine whether stents are needed or if patients can be treated medically. However, use of FFR has been stymied by the cost of the pressure wires, the need to use adenosine and the additional time it takes. Three companies (Medis, CathWorks and Philips) are now working on angiography imaging-based FFR technology that can assess lesions using imaging and contrast and create a tableside 3-D reconstruction of the vessel segment with a color-coded overlay showing virtually derived FFR readings. This would eliminate the need for pressure wires and offer better anatomical guidance if a stent is necessary. Two TCT late-breaking trial presentations involved use of the Medis Quantitative Flow Ratio (QFR) technology. See a video example of this technology. CathWorks also announced it is beginning a U.S. clinical trial (FAST-FFR) for its technology, which will be headed by William Fearon, M.D., director of interventional cardiology, Stanford University Medical Center. I spoke to Fearon at TCT on this technology in this video interview.
Creation of the National Cardiogenic Shock Initiative
Henry Ford Hospital partnered with several hospitals in Detroit to create the Detroit Cardiogenic Shock Initiative where new protocols were implemented in attempts to reduce mortality from this complication of acute myocardial infarction (AMI) that affects approximately 5-8 percent of U.S. heart attack patients. The protocol used immediate implantation of the Abiomed Impella percutaneous heart pump in shock patients before revascularization, resulting in a 50 percent drop in deaths. After the first report at ACC.17, Henry Ford Hospital fielded calls from cardiology departments around the country about starting similar programs. At TCT, it was announced Henry Ford would head a new National Cardiogenic Shock Initiative, working with hospitals across the U.S. to expand this protocol and to maintain a registry of data on patients treated. I attended a dinner with leaders of several hospitals in this new initiative and spoke with William W. O’Neill, M.D., medical director, Center for Structural Heart Disease, Henry Ford Hospital, Detroit, about the program. Watch a video of the interview.
Expanding Use of New Technologies, Techniques for Complex Lesions
Over the past two years there has been an increasing focus on how interventional cardiologists can tackle complex lesions and total chronic occlusions (CTOs) in so-called complex high-risk patients (CHIP). TCT has expanded sessions covering CHIP topics and created several CHIP workshops throughout the year. Experts in CHIP procedures say the technologies to treat these complex patients have improved to the point where most interventional operators can learn to treat these lesions if the training is available. As interventional patient volumes decrease, this is a new avenue for cath labs to consider, especially for patients who do not want or do not qualify for surgery.
I spoke with two key thought leaders in this area of treating CHIP patients who presented at both TCT and ACC. Watch my interviews with Bill Lombardi, M.D., director of complex coronary artery interventions at the University of Washington, and Farouc Jaffer, M.D., Ph.D., director of coronary interventions at Massachusetts General Hospital.
Other New Cardiovascular Technologies
I created two videos that focus on the bleeding-edge cardiovascular technologies highlighted at TCT 2017. I spoke with Juan Granada, M.D., Cardiovascular Research Foundation president and chief executive officer, who shared his insights on what technologies he predicts will become mainstream therapies in the coming years in the video “Cath Lab of the Future Cardiovascular Technologies to Watch.” Some of the most interesting new technologies highlighted on the exhibit hall floor can be found in the video “Editor's Choice of the Most Innovative New Technologies at TCT 2017.”



 October 24, 2025
October 24, 2025