September 10, 2015 — The Carillon mitral contour system is projected to be a cost-effective treatment option when compared to a typical regimen of optimal medical treatment (OMT), the present standard of care for functional mitral regurgitation (FMR). This finding has been established in a new study published in BMC Cardiovascular Disorders.
Researchers analyzed data derived from the landmark TITAN clinical trial, which demonstrated significant clinical merits of the Carillon technology, and developed a comprehensive cost-effectiveness model that projects the costs of the technology and compares them to the benefits. The output shows the Carillon therapy to be cost-effective in comparison to OMT with an incremental cost-effectiveness ratio (ICER) of €15,533 per quality-adjusted life year (QALY).
ICER is considered to be a gold standard in terms of determining whether a new therapy is cost-effective, with amounts below €35,000/QALY typically considered to be cost effective. The analysis was conducted from the perspective of the German health insurance system.
“In multiple clinical trials, Carillon has demonstrated significant reductions in FMR and improvement in heart failure symptoms. This analysis takes the next step in projecting that these benefits result in the cost effectiveness of the therapy,” said Michael Haude, M.D. Ph.D., chief of cardiology at Lukaskrankenhaus in Neuss, Germany, and co-author of the study. “This positive assessment has substantial implications in terms of the utility and accessibility of the therapy.”
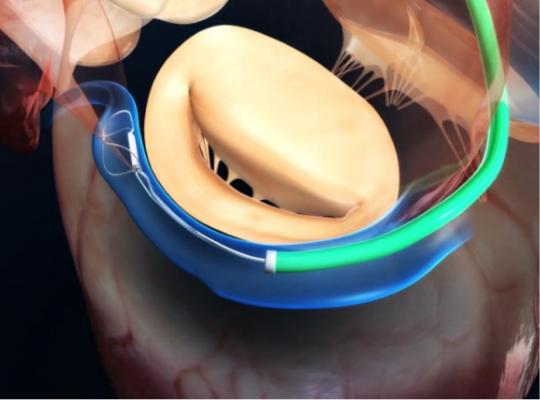
The Carillon mitral contour system is a percutaneous mitral annuloplasty treatment option that can be deployed rapidly and safely, utilizing standard interventional techniques. The implantable device consists of a distal anchor and a proximal anchor connected by a shaping ribbon and utilizes the heart’s venous anatomy to reshape the mitral annulus. This approach allows for reduction of the dilated annulus, addressing a root cause of FMR. The system has demonstrated compelling efficacy, significantly improving patients’ symptoms, mitral regurgitation and quality of life. In addition, all adjunctive treatment options remain available after using Carillon, making it an ideal first-line therapy for FMR.
For more information: www.cardiacdimensions.com


 July 08, 2024
July 08, 2024 









