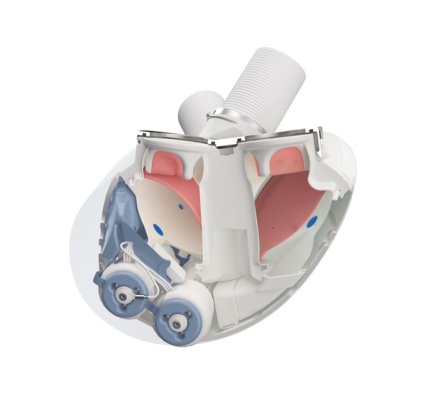
Aeson total artificial heart showing Open view with pumps and electronics (blue), blood chambers (maroon) and conduits (top, white). Image courtesy of Carmat.
March 28, 2022 — Carmat, the designer and developer of the world’s most advanced total artificial heart, aiming to fulfill an unmet medical need by providing a therapeutic alternative to people suffering from end-stage biventricular heart failure, issues an update on its activities and outlook.
Restart of production and confirmation of the objective of resuming implants in October 2022
Following the characterization of the quality defects on the two components that were the root cause of issues affecting some of its prostheses, Carmat, in close collaboration with its suppliers, defined the necessary corrective actions.
These actions have now been implemented within the production processes of the relevant suppliers, and notably include additional controls.
Given supply and production lead times for suppliers and at Carmat’s plant in Bois-d’Arcy, the company can thus confirm that new implantable prostheses will be available in October 2022.
At the same time, the Company is continuing its discussions with the notified body (DEKRA) and the competent authorities (the ANSM in France and the Food & Drug Administration in the United States), whose authorization is required to resume implants.
These elements allow Carmat to confirm its objective of a resumption in commercial and clinical implants in October 2022.
Ongoing training for hospitals
Due to the lack of therapeutic solutions available to patients with end-stage heart failure, Carmat is seeing strong demand for the Aeson system from cardiologists, notably driven by positive feedback from physicians who have already implanted the device.
Carmat is thus continuing to train more hospitals, in Germany and in other European countries, in order to be able to meet, to the extent possible, this demand once the suspension of implants is lifted.
Stéphane Piat, Chief Executive Officer of Carmat, concluded: “I would like to pay tribute to the strong mobilization of Carmat’s teams who, by providing our suppliers with their assistance and expertise, have enabled the changes necessary to improve the sturdiness and reliability of the components that led to the incidents we experienced in 2021 to be rapidly implemented within manufacturing processes. Thanks to this close collaboration, the resumption of production, incorporating these changes, is now effective.
This allows us, given our supply and production lead-times, to confirm our objective of providing medical teams with implantable prostheses from October 2022, thus enabling our implants to resume at that time, subject of course to the approval of the notified body DEKRA and the competent authorities with whom we are continuing our constructive dialogue.
Given the lack of satisfactory therapeutic solutions addressing advanced heart failure and the number of patients on a waiting list for a heart transplant, we are receiving requests from many physicians and are doing everything we can to be able to resume our implants as soon as possible while continuing to prioritize the health and safety of patients."
For more information: https://www.carmatsa.com/


 February 03, 2026
February 03, 2026 









