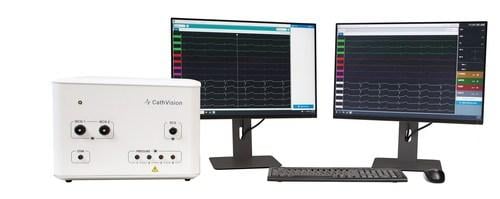
The ECGenius System is an innovative electrophysiology (EP) recording system designed to guide and enhance ablation therapy through the acquisition of low-noise, high-fidelity EP signals.
April 27, 2022 – CathVision, a medical technology company developing innovative electrophysiology solutions designed to guide and enhance cardiac ablation therapy through the acquisition of low-noise, high-fidelity electrograms, announced the completion of patient enrollment in the CathVision ECGenius System clinical evaluation study at the University of Vermont Medical Center. The study is the first in the U.S. to evaluate the safety and technical performance of ECGenius and benchmark electrogram signal quality compared to commercially available systems.
Conventional EP recording systems typically acquire suboptimal quality electrogram signals, preventing the accurate analysis and interpretation of those signals and severely limiting the ability of electrophysiologists to correctly diagnose and devise ablation strategies for complex arrhythmias including atrial fibrillation (AF). ECGenius represents an important evolution in the quality of ECG signal acquisition, interpretation and therapy support.
"What we've seen so far clinically with ECGenius are sharper, higher frequency signals compared to the systems that have been in our labs for years, and without a notch filter," said Principal Investigator Dr. Nathaniel Thompson, M.D., University of Vermont Medical Center, Burlington, Vermont. "The noise level with ECGenius is quite low and has allowed our team to clearly visualize very precise cardiac signals – including His – that are normally blurred or rendered completely undetectable because of the baseline noise associated with using more conventional recording systems."
ECGenius can be seamlessly integrated into modern hospital environments and features a 12-lead ECG, 128 intracardiac channels and four blood pressure channels. Additionally, the system is compatible with existing catheters and 3D mapping systems.1
"EP recording systems have seen almost no meaningful evolution in decades. This lack of innovation has created an unacceptable status quo that curbs the advanced diagnosis and treatment of complex cardiac arrhythmias. ECGenius is changing that," said Mads Matthiesen, CEO, CathVision. "ECGenius delivers a modern approach built on improved visualization and higher quality raw data that paves the way for more informed and accurate decision-making processes in the EP lab. This is necessary, foundational change and will become the basis for artificial intelligence-driven therapy support and machine learning software tools."
CathVision is actively partnering with additional clinical sites to evaluate ECGenius and aid in the development of AI-based solutions that can be integrated into the system. For more information about evaluating ECGenius, please visit www.cathvision.com/ep-recording-enhanced or reach out to [email protected].
The ECGenius System is not currently approved for commercial use in Europe or the United States.
1A list of compatible devices can be found in the ECGenius IFU.
For more information: https://cathvision.com/


 January 05, 2026
January 05, 2026 









