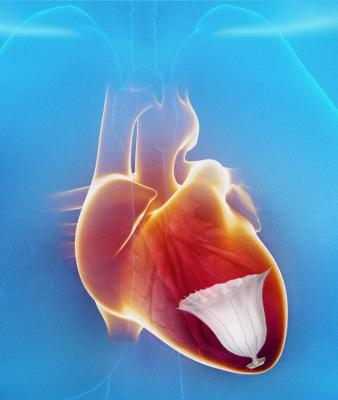
May 28, 2013 — CardioKinetix Inc. has announced the results of a meta-analysis study of the catheter-based Parachute Ventricular Partitioning Device. Six-month clinical results from 91 U.S. and European patients with ischemic heart failure were presented.
“The results of this meta-analysis, which represents the largest group of patients studied to date, are consistent with previous positive findings and continue to substantiate the Parachute treatment as a viable technology,” said Martyn Thomas, M.D., chairman of Cardiology at St. Thomas Hospital in London, England. “The Parachute promises to revolutionize the treatment for heart attack survivors whose hearts enlarge over time and subsequently suffer from heart failure symptoms, offering hope for a better qualify of life for many patients.”
Clinical data from 91 patients to reach six-month followup demonstrates successful delivery and deployment of the Parachute implant, without the occurrence of major adverse cardiac events (MACE) related to the device, in 90 percent (82/91) of patients at six months following treatment. In addition, six months following treatment, 89 percent of patients demonstrated improved or maintained New York Heart Association (NYHA) functional class status. Specifically in the NYHA III subgroup, 27 percent improved two classes. The treatment also demonstrated a reduction in left ventricular volume, with a 20 percent reduction in end diastolic volume.
After a heart attack, many patients experience enlargement of their left ventricle causing a decrease in cardiac output resulting in heart failure symptoms such as shortness of breath. Treatment options for patients whose ventricle has enlarged are limited. The Parachute device offers the first minimally invasive catheter-based treatment to partition the damaged muscle, excluding the non-functional heart segment from the healthy, functional segment to decrease the overall volume of the left ventricle and restore its geometry and function.
For more information: www.cardiokinetix.com


 July 08, 2024
July 08, 2024 









