February 5, 2015 — A new computer program allows doctors to assess blood flow as they are using flow-diverter devices to treat life-threatening aneurysms, suggests a preliminary study being presented at the 27th annual International Symposium on Endovascular Therapy (ISET).
“Until now, there was no safe way to measure the blood flow in real-time, during the procedure, including flow reduction to the aneurysm and flow to the rest of the brain,” said Aichi Chien, Ph.D., assistant professor of interventional neuroradiology at the University of California, Los Angeles. “Because the program quantifies blood flow automatically, doctors do not need to stop the procedure to get this information, which helps them make the best decisions during the procedure.”
A flow diverter is a small stent made of a fine mesh that is implanted minimally invasively in an artery at the site of an aneurysm. A flow diverter redirects blood past the aneurysm to remove the pressure that could cause the aneurysm to rupture, and the patient to potentially bleed to death. Flow diverters typically are used to treat large (2-2½ centimeters) or giant (more than 2½ centimeters) aneurysms, or those with wide necks.
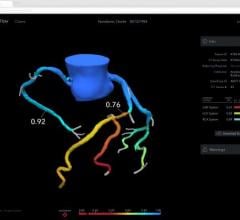
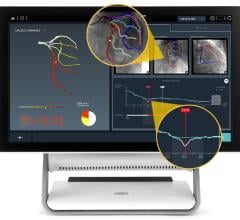
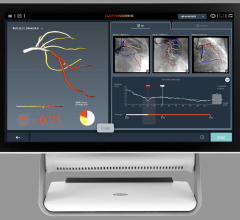
The Intracranial Stent Flow Mapping computer program — IS FlowMap — takes advantage of the standard digital subtraction angiography (DSA) imaging being taken during the procedure. The program analyzes the standard images, compares the difference in blood flow image to image and calculates the changes within seconds, providing that information to the doctor during treatment. If blood flow isn’t optimal, the doctor may choose to use a different treatment, such as placing coils in the aneurysm.
In the study, 13 patients were treated, and the average reduction in blood flow entering the aneurysm was 48 percent. The flow diverter healed the aneurysm in 11 (85 percent) of the patients.
While 3 to 5 million people in the United States have brain aneurysms, most don’t cause problems. About 30,000 people a year suffer from a ruptured aneurysm. Anyone can develop an aneurysm, but risk factors include high blood pressure, alcohol and drug abuse and smoking.
“There are many advances in devices to treat patients with aneurysms and other vascular disease, but the technology to see the effects of those devices is very limited,” said Chien. “The IS FlowMap provides a simple way to analyze the treatment without any additional procedures or risk. We’ll be able to use this information moving forward to compare treatment and determine what amount of blood flow change is optimal.”
For more information: www.iset.org


 February 03, 2026
February 03, 2026