June 28, 2023 — CathWorks announced that the first patient has been enrolled in the Advancing Cath Lab Results with FFRangio Coronary Physiology Assessment(ALL-RISE) Study. The ALL-RISE Study is a prospective randomized controlled trial (RCT) aimed at transforming the diagnosis and treatment of coronary artery disease (CAD) by evaluating the clinical and economic benefits of the CathWorks FFRangio System.
The study is expected to enroll 1,924 patients at up to 60 participating sites in North America, Asia, Europe and the Middle East. Patients presenting with coronary lesion(s) will be randomized to FFRangio-guided treatment or invasive pressure wire-guided treatment. While this is a global study, it will be the first RCT to assess outcomes of an angiography-based physiological assessment tool with patients enrolled in the United States.
The ALL-RISE Study is led by Study Chair, Dr. Ajay J. Kirtane (NewYork-Presbyterian/Columbia University Irving Medical Center) and Principal Investigators, Dr. William Fearon (Stanford University) and Dr. Allen Jeremias (St. Francis Hospital & Heart Center), and the first patient was enrolled by Dr. Amir Kaki (Ascension St. John Hospital). "We have been utilizing the FFRangio System for more than a year to better guide treatment decisions for patients with CAD and have adopted it as our physiology assessment tool of choice due to the safety and efficiency benefits it offers," said Dr. Kaki. "We, at Ascension St. John Hospital, are pleased to play a pivotal role in the ALL-RISE Study and hope our findings will enable more physicians to experience the same benefits we have in our lab since adopting FFRangio."
"The enrollment of the first patient in the ALL-RISE Study marks an important step forward in our collective efforts to enhance precision-based medicine to optimize efficacy of CAD interventions," said Dr. Martin B. Leon, Professor of Medicine at Columbia University Irving Medical Center, Director of Columbia Interventional Cardiovascular Care and Chairman of the ALL-RISE Executive Committee. "We are grateful for the collaboration of leading institutions and clinical investigators around the globe who will be generating clinical and economic evidence that will shape the future of percutaneous coronary interventions."
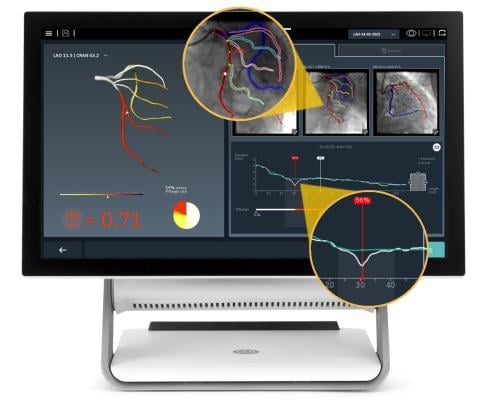
The FFRangio System combines artificial intelligence (AI) and advanced computational science to obtain quick and reliable FFRangio values from routine angiograms (X-rays), eliminating the need for drug stimulation and invasive pressure wires. The FFRangio System has the potential to enhance patient outcomes, improve workflow efficiency and reduce costs for healthcare systems.
"We are incredibly excited to reach this significant milestone for CathWorks," said Ramin Mousavi, President and Chief Executive Officer of CathWorks. "The ALL-RISE Study reinforces our commitment to advancing the interventional cardiology field by providing additional evidence to the clinical community on the potential impact of FFRangio in transforming patient care."
In July 2022, CathWorks entered into a strategic partnership with Medtronic to amplify its efforts in bringing the benefits of FFRangio to more physicians and patients globally. The partnership underscores Medtronic's commitment to investing in innovative technologies that support physicians and patients from diagnosis to treatment.
"Through our partnership with CathWorks, we have been able to see firsthand how this new, innovative technology helps to advance diagnosis, treatment and management of CAD," said Jason Weidman, Senior Vice President and President of the Coronary and Renal Denervation Operating Unit at Medtronic. "We look forward to seeing how this important study adds to the existing evidence on the benefits of FFRangio and continues to drive adoption of the technology in clinical practice."
For more : www.clinicaltrials.gov and www.cath.works
Related FFR-angio Content:
CathWorks Presents New Data and Latest Innovations at ACC 2022
CathWorks FFRangio System Receives National Reimbursement Approval in Japan
VIDEO: Angiography Image-based FFR May Eliminate Need for Pressure Wires — Interview with William Fearon, M.D.
Novel FFR Methods Can Reduce Procedure Time and Cost
CathWorks FFRangio System Receives U.S. FDA Clearance
VIDEO: Example of the Medis QFR Angiography Image Derived FFR System
Medis QAngio XA 3D Receives FDA 510k Clearance
Image-based FFR May Replace Pressure Wires and Adenosine
CathWorks Receives CPT Code for Non-invasive FFRangio Measurements During PCI
Find more news and new technology in the FFR Technology Channel


 January 05, 2026
January 05, 2026 









