Getty Images
September 28, 2021 — A research team at the University of Arkansas for Medical Sciences (UAMS) has identified a potential cause of long-lasting symptoms experienced by COVID-19 long-hauler patients. They found an antibody creates problems for the immune system by attacking the angiotensin-converting enzyme 2 (ACE2).
The findings were published in the journal Public Library of Science ONE (PLOS ONE).[1]
At the heart of the team’s findings is an antibody that shows up weeks after an initial infection and attacks and disrupts a key regulator of the immune system, said lead researcher John Arthur, M.D., Ph.D., professor and chief of the Division of Nephrology in the UAMS College of Medicine, Department of Internal Medicine.
The antibody creates problems for the immune system by attacking the angiotensin-converting enzyme 2 (ACE2). The ACE2 enzyme helps regulate the body’s response to the virus by metabolizing a peptide that activates the immune system. The attacking antibody interferes with ACE2’s work, which makes the antibody a prime suspect for the long-lasting illness.
As many as 30% of COVID-19 patients experience lingering fatigue, brain fog and shortness of breath. The cause of long COVID-19 has eluded scientists, but the UAMS team’s discovery sheds important new light on the molecular-level mechanisms behind it.
“Everything that we’ve found is consistent with this antibody as the instigator of long COVID, so it’s an exciting development that merits further study,” Arthur said.
The research team was brought together quickly this spring by the UAMS Translational Research Institute to test the hypothesis that developed through discussions between Arthur and UAMS’ Terry Harville, M.D., Ph.D., a professor in the Department of Pathology and medical director of the Histocompatibility and Immunogenetics Laboratories.
Researchers Karl Boehme, Ph.D., Craig Forrest, Ph.D., and Shana Owens, Ph.D., in the Department of Microbiology and Immunology developed the assay (test) used to identify the two antibodies.
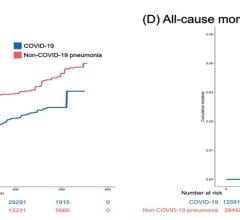
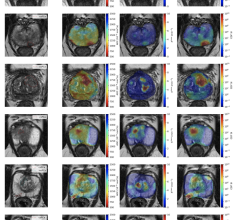
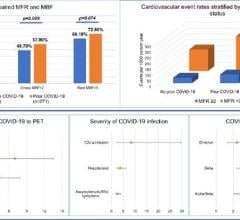
Researchers tested plasma or serum for ACE2 antibodies in 67 patients with known SARS-CoV-2 (the virus that causes COVID-19) infection and 13 with no history of infection. In 81% of blood samples from patients in Arkansas and Oklahoma with a history of COVID-19, the samples had the antibody that attacked the ACE2.
In participants with no history of COVID-19, no antibodies were created to attack the ACE2 enzyme.
“If we show that the whole hypothesis is right, that this interference of ACE2 really does cause long COVID, then it opens up many potential treatments,” Arthur said. “If our next steps confirm that this antibody is the cause of long COVID symptoms, there are medications that should work to treat them. If we get to that phase of research, the next step would be to test these drugs and hopefully relieve people of the symptoms they're having.”
The multidisciplinary team also includes College of Medicine researchers Christian Herzog, Ph.D., Department of Internal Medicine; Josh Kennedy, M.D., Department of Pediatrics; and Juan Liu, Ph.D., from the Department of Pathology.
“This is true team science,” Arthur said. “We put together a great group of investigators that had never worked together to produce these very exciting results.”
For more information: www.uams.edu or www.uamshealth.com
Related Cardiology Related COVID-19 Content:
Large Study Confirms Statins Reduce COVID-19 Severity
First Randomized Trial Backs Safety of ACE and ARB Heart Drugs in COVID-19 Patients
No Evidence Supporting Discontinuing RAAS Inhibitors in COVID-19 Patients
VIDEO: What Cardiologists Need to Know about COVID-19 — Interview with Thomas Maddox, M.D.
The Cardiac Implications of Novel Coronavirus
ESC Council on Hypertension Says ACE-I and ARBs Do Not Increase COVID-19 Mortality
AHA Explains Severe COVID-19 is Closely Associated With Heart Issues
Reference:

 March 20, 2024
March 20, 2024