June 22, 2015 - The current monitoring of patients with cardiac implantable electronic devices (CIEDs) may be underestimating device problems, according to UC San Francisco researchers. They propose systematic methods to determine accurate causes of sudden death in those with CIEDs such as defibrillators and pacemakers, as well as improved monitoring for device concerns.
Their study appears online June 22 in JAMA Internal Medicine.
"With a vast majority of out-of-hospital sudden deaths evaluated by medical examiners or coroners, CIED problems are often missed in the postmortem investigation, as they are not part of the routine evaluation," said lead author Zian H. Tseng, M.D., MAS, associate professor of medicine in residence in the Cardiology Division and Cardiac Electrophysiology Service at UCSF. "We believe the findings may have broader implications for Food and Drug Administration post-market surveillance of CIEDs and physician practice."
More than 3 million Americans have a permanent pacemaker or implantable cardioverter-defibrillator (ICD), according to the researchers. Current surveillance for CIED malfunctions is based on the U.S. Food and Drug Administration-mandated Manufacturer and User Facility Device Experience database, which is mandatory for manufacturers but voluntary for healthcare professionals.
Regulations require manufacturers and facilities such as hospitals report when they learn a device may have caused or contributed to a serious death or injury. However, because more than 90 percent of sudden cardiac deaths occur outside the hospital and autopsies of sudden deaths from CIEDs are rarely performed, the causes and incidence of device failure in sudden deaths are unknown.
To examine the possible causes, researchers led by Tseng conducted a prospective autopsy study of all sudden deaths with pacemakers or defibrillators over a 35-month period as a sub-study of the San Francisco POstmortem Systematic InvesTigation of Sudden Cardiac Death (POST SCD) Study. POST SCD aims to discover for the first time the true causes of sudden cardiac death, why it is more prevalent in some demographic populations and whether it is too often inaccurately cited as a cause of death.
Working with senior author Ellen Moffatt, M.D., in the City and County of San Francisco Office of the Chief Medical Examiner, UCSF is fully investigating every death attributed to a sudden cardiac event in San Francisco over a five-year period. They hope to learn the biological risk factors for the disease and improve prevention, medical and interventional therapy for patients.
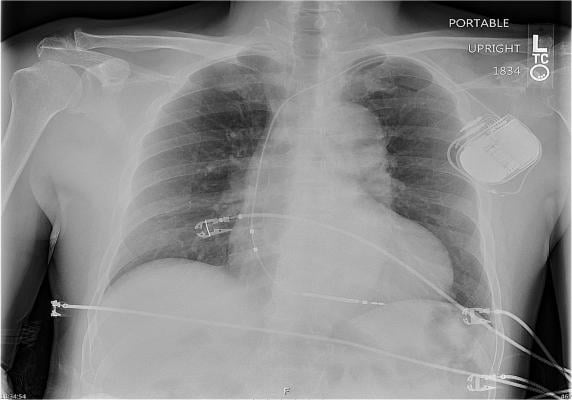
Of 517 sudden deaths investigated in the JAMA Internal Medicine study, 22 (4.3 percent) had CIEDs. The autopsies revealed a non-cardiac cause of death in six of the 22. Six of the 14 pacemaker sudden deaths and seven of eight defibrillator sudden deaths were from ventricular tachycardia (VT, very rapid heartbeat originating in the ventricles) and ventricular fibrillation (VF, disorganized, ineffective ventricle contractions).
Device concerns were identified in exactly half of the 22 sudden deaths – four pacemakers and seven defibrillators. Three were hardware failures, five were defibrillators with VF undersensing, one was a defibrillator with VT missed due to programming, one was an improper device selection, and another was a pacemaker-dependent patient with pneumonia and concern for lead fracture.
Although defibrillators in general are highly effective at treating VT/VF, the researchers found that six of eight ICD sudden deaths had either undersensing of ventricular arrhythmias or hardware failure. One of the ICDs missed events that required rescue by external defibrillators.
Separately, the team identified and reviewed the records of all 712 San Francisco residents with a defibrillator during the three-year study period. Overall, 109 (15.3 percent) died, and the seven defibrillator issues represented 6.4 percent of these deaths.
These study findings hopefully will raise awareness among medical examiners and coroners to consider interrogation and autopsy in their determination of causes of sudden deaths with CIEDs, Moffatt said.
"Because these devices are intended to prevent sudden death, careful monitoring for potential device problems should include a complete postmortem investigation when sudden deaths occur in those with CIEDs," Tseng said. "It is also important to recognize that we found that a third of device concerns were related to physician practices and present opportunities for practice improvement."
For more information: www.ucsfhealth.org


 September 16, 2025
September 16, 2025