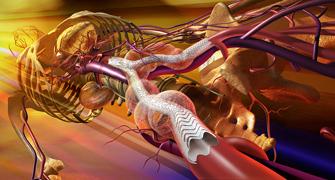
September 17, 2010 - Clinical uses will soon begin for the C3 Delivery System for the Gore Excluder Device as a minimally invasive treatment for patients suffering from an abdominal aortic aneurysm (AAA). The delivery system, made by W.L. Gore & Associates, provides surgeons with cannulation options and allows them to bring the contralateral gate to the contralateral guidewire.
Dr. Eric Verhoeven, Chief, Department of Vascular and Endovascular Surgery, at Klinikum Süd in Nürnberg, Germany, performed the first procedure to treat a patient with an abdominal aortic aneurysm (AAA) using the new system. Additional procedures were performed subsequently by Dr. Piotr Kasprzak, Leader of Vascular Surgery, at Klinikum der Universität in Regensburg, Germany and Dr. Hans-Joachim Florek, Chief of Vascular Surgery, at Elbe-Weißeritztal Kliniken in Dresden (Freital), Germany.
Once delivered into the aorta, the Gore C3 Delivery System enables repositioning of the stent graft. The ability to reposition the device can minimize complications that could occur if the graft is positioned incorrectly during the initial deployment.
“We specifically selected our initial patients with short or angulated necks, or with difficult iliac anatomy, to be able to use the graft to its full extent," Verhoeven said. "In the four patients treated with the Gore C3 Delivery System, we repositioned the graft twice for level and for rotational orientation, to maximize the sealing zone in the aortic neck and facilitate cannulation of the contralateral limb. In the other two patients repositioning was unnecessary because either the proximal position was perfect after the initial deployment, or cannulation was immediately successful. However, having the option to reposition the Gore Excluder Device enabled us to treat these challenging anatomies with added confidence during our initial deployment. Overall, the Gore C3 Delivery System worked as it was designed and provided unparalleled control while remaining intuitive and easy to use.”
More than 100,000 Gore Excluder Devices have been implanted in patients worldwide since 1997. The Gore Excluder AAA Endoprosthesis is constructed of a durable ePTFE bifurcated graft with an outer self-expanding nitinol support structure which provides both device flexibility and material durability. The function of the endoprosthesis is to internally reline the abdominal aorta, including the bifurcation, and isolate the aneurysm from blood circulation. The device is inserted by a catheter-based delivery technique through small incisions in the patient’s legs.
For more information: www.goremedical.com


 April 26, 2023
April 26, 2023 









