September 28, 2010 – The Transcatheter Cardiovascular Therapeutics (TCT) 2010 symposium featured a live case broadcast of a physician implanting a new device to treat refractory angina.
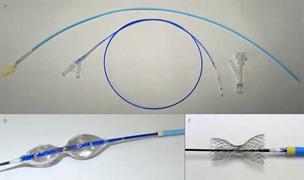
At the Antwerp Cardiovascular Institute/ZNA Middelheim in Belgium, Stefan Verheye, M.D., successfully implanted the Neovasc Reducer in the patient’s coronary sinus. Verheye is the principal investigator in the COSIRA study, designed to assess the efficacy of Neovasc’s implantable Reducer device as a treatment for refractory angina.
Refractory angina is a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate blood supply to the heart muscle.
The reducer is implanted in the coronary sinus vein using a minimally invasive percutaneous procedure that is similar to implanting a coronary stent and takes about 20 minutes. It is intended to provide relief by altering blood flow to the coronary sinus, thereby increasing perfusion of oxygenated blood to certain areas of the heart muscle
For more information: www.neovasc.com


 January 05, 2026
January 05, 2026 









