March 1, 2010 – A collaborative agreement with the Universidad San Francisco de Quito in Ecuador and Vicor Technologies Inc. to help identify patients at risk of sudden cardiac death and autonomic nervous system dysfunction.
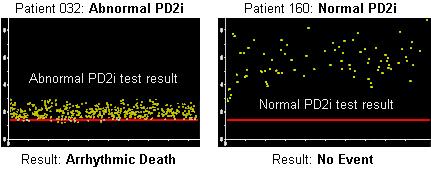
The agreement uses Vicor Technologies noninvasive medical devices and diagnostics using its PD2i nonlinear algorithm and software. The its PD2i Analyzer displays and analyzes electrocardiographic data to provide a measure of heart rate variability (HRV) in patients at rest, and during controlled exercise and paced respiration. The clinical significance of HRV is physician determined.
Under the terms of the agreement Vicor will collaborate with the Universidad San Francisco de Quito (USFQ) to develop academic programs that advance awareness and use of the measure of heart rate variability (HRV) to diagnose various medical disorders within a variety of health-challenged and/or at risk populations. It will also promote continued research into the uses of HRV in USFQ clinics, and stimulate assistance from Ecuador’s public and private health sectors. The goal is to prevent disease with appropriate intervention/treatment based on early diagnosis using HRV.
Additionally, Vicor will serve as the sponsor of the Vicor Technologies Inc. Heart Rate Variability Institute at the Universidad San Francisco de Quito, with the express purpose of furthering throughout Ecuador HRV research and its use as a medical diagnostic as measured with Vicor’s PD2i Analyzer.
Specifically, this collaboration will involve the Inclusion Institute, its emergency rooms, and intensive care units for applications involving patients at imminent risk of death, those with severe psychiatric illness, and those with elevated cardiovascular risk and mental illness.
This effort may also include study of HRV as measured by Vicor’s PD2i Analyzer to identify autonomic nervous system dysfunction in diabetics, the need for lifesaving intervention in trauma victims as a means of facilitating triage efforts, imminent mortality in those with brain injury, and the risk of sudden cardiac death in target populations, including young athletes.
“Science and technology have propelled knowledge and practical applications in medicine at a fantastic speed. The collaboration of several universities for the purpose of investigating the risk of sudden death in certain special groups will definitely broaden the spectrum of routine procedures that will result in the prevention of these events and the salvage of precious human lives,” said Dr. Enrique Noboa, dean, USFQ School of Medicine.
“Autonomic nervous system dysfunction and its role in disease constitute a field of continuous expansion and research,” said Dr. Michelle Grunauer, project manager, USFQ HRV Institute. “The implications of heart rate variability analysis involve several disciplines, and focus our attention in multi-causality. Furthermore, the rapid, precise, noninvasive and cost-effective stratification of risk of AD in patients admitted to the Emergency Department with the PD2i test is invaluable.”
The Universidad San Francisco de Quito is a nonprofit liberal arts university. Founded in 1988 by the Corporación de Promoción Universitaria, (its umbrella nonprofit foundation), the USFQ was the first totally private, self-financed university in Ecuador. Today, the USFQ has 16 colleges and academic institutes spread across its main campus in Cumbayá-Quito and The Galapagos Academic Institute for the Arts and Sciences in San Cristobal in the Galapagos Archipelago. Additionally, USFQ has a biodiversity research station in Tiputini and an anthropology research institute in Riobamba.
For more information: www.vicortech.com


 January 05, 2026
January 05, 2026 









