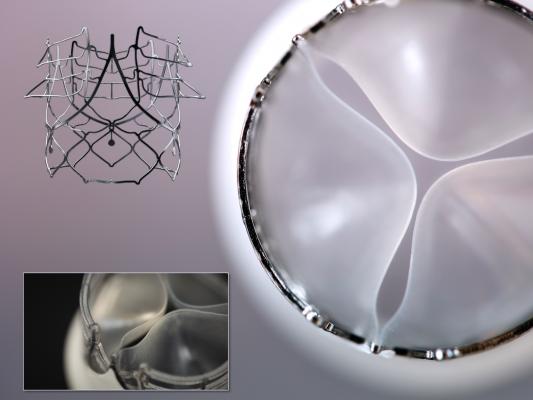
June 4, 2019 — Royal DSM recently announced a collaboration with Strait Access Technologies (SAT), to develop the world’s first durable and cost-effective transcatheter replacement heart valves with polymeric leaflets.
This collaboration is initially focused on developing valves to treat rheumatic heart disease (RHD), a condition that affects more than 30 million people globally, mainly in emerging markets. RHD was declared a global health priority by the World Health Organization (WHO) in a special resolution in 2018. This resolution recognizes that up to now, efforts to prevent and treat RHD have been unsuccessful. Existing mechanical or animal-tissue replacement valves are unsuited for the challenging pathologies of typically-younger RHD patients. In addition, lack of suitable medical facilities and high valve costs effectively condemn the majority of patients living with RHD to a premature death.
SAT and DSM are collaborating to produce products that address the unique biological, cost and access challenges posed by RHD. The replacement heart valves being developed by SAT and DSM will be based on platform technologies developed by SAT in recent years. At their core, the valves will use DSM’s advanced CarboSil Thermoplastic Silicone-Polycarbonate-urethane (TSPCU) biomedical polymers, negating the need for invasive open-heart surgery or the lifelong burden of anticoagulant drugs. In a second phase, these polymeric leaflet valves will be offered to patients in developed economies suffering heart valve failure associated with age, diet and other factors.
For more information: www.dsm.com, www.straitaccesstechnologies.com


 November 14, 2025
November 14, 2025 









