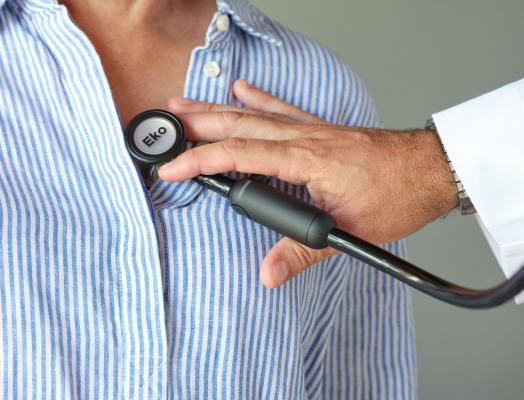
December 30, 2019 — Eko, a digital health company with an AI-powered cardiac screening platform, announced the second generation of its CORE product line. An evolution from Eko's first-generation CORE, the new CORE features powerful amplification and active noise cancellation that enables doctors and nurses to hear heart and lung sounds with greater clarity and screen patients for heart disease, the number one cause of death in the U.S., more effectively.
“When heart and lung sounds are amplified in the screening of heart valve and pulmonary diseases, everything changes,” said Connor Landgraf, co-founder and CEO of Eko. “Our mission at Eko continues to be to enable all healthcare professionals to provide the highest level of cardiac care through non-invasive, reliable products. The redesigned CORE puts the ears of a trained cardiologist in the hands of any doctor or nurse.”
Two hundred years after its invention, the stethoscope is still the most heavily relied upon cardiac and pulmonary screening tool. With a sleeker design, unprecedented active noise cancellation, and forthcoming decision support algorithms, the second-generation CORE will elevate what clinicians already use in every physical exam and close gaps in care created in part by analog stethoscopes.
“The better we can hear and the better we can screen, the better we can care for our patients,” said Dr. Steve Pham, VP of clinical research at Eko and an emergency medicine physician. “The CORE, coupled with Eko’s software, helps convert the device already worn around the neck of 30 million clinicians around the world into a powerful cardiopulmonary screening tool.”
Since releasing its first-generation CORE in 2015, clinicians at over 4,000 hospitals have used the device to enhance their practice.
The second-generation CORE will be available on Eko’s website to healthcare providers as an attachment to their existing stethoscope (sold as the CORE Digital Attachment) or as a fully assembled digital stethoscope (sold as the CORE Digital Stethoscope) for $199 and $249, respectively.
CORE Digital Attachment and CORE Digital Stethoscope features include:
- Active noise-cancellation, perfect for honing in on heart and lung sounds in loud settings
- 40x sound amplification with seven volume settings, for enhanced clarity of critical sounds
- Easy toggling between acoustic and digital modes
- Lithium-ion battery with 10-hour life and micro-USB charging
- Wireless Bluetooth connection to Eko’s software
- Save 15, 30, 60 or 120-second recordings with “one click” recording button
Eko’s software allows users to capture, analyze and share sound data:
- View sound waveforms and phonocardiograms
- Eko’s screening algorithms, currently FDA-pending, will assist clinicians in analyzing heart sounds for pathologic murmurs and valvular heart diseases
- Share recordings with colleagues for a second opinion or live stream cardiology-grade sounds with an Eko Enterprise plan, which powers reliable real-time connections between clinicians and patients, or between clinicians for second opinions
Eko’s screening algorithms are in development and have not been cleared by the FDA.
For more information: www.ekohealth.com


 November 12, 2025
November 12, 2025 









