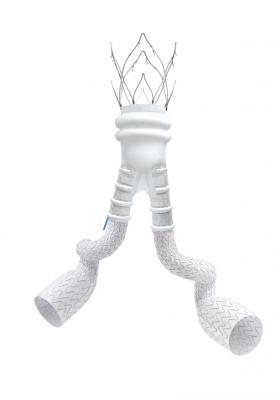
February 6, 2018 – Endologix, a developer and marketer of treatments for aortic disorders, announced the completion of enrollment in the Expanding Patient Applicability with Polymer Sealing Ovation Alto Stent Graft (ELEVATE) IDE clinical study. The objective of the 75-patient study is to evaluate the safety and effectiveness of the Alto Abdominal Stent Graft System for the repair of infrarenal abdominal aortic aneurysms (AAAs). The company plans to file regulatory submissions in the third quarter of 2018 and anticipates potential approval of the Alto device in both the U.S. and European markets in 2019.
The study’s principal investigator, Sean Lyden, Chairman of the Department of Vascular Surgery at Cleveland Clinic, commented, "We are pleased to complete enrollment of the ELEVATE IDE Trial and look forward to evaluating the clinical results later this year. The Alto device incorporates several design enhancements that are intended to simplify the procedure and enable the system to treat a wider range of AAA anatomies than Ovation iX.”
Alto is the latest-generation Polymer EVAR system, which expands patient applicability by moving the polymer sealing ring near the proximal edge of the graft. The new device was designed based upon physician feedback and the positive clinical results from the Ovation platform that has been extensively studied in over 1,300 patients from five prospective studies over the past seven years. Alto is an investigational device not currently approved in any market, and its safety and effectiveness have not been established.
For more information: www.endologix.com


 January 05, 2026
January 05, 2026 









