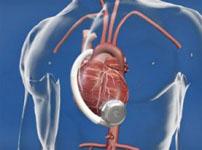
June 16, 2010 – A ventricular assist system has received conditional approval from the U.S. Food and Drug Administration (FDA) to begin a new clinical trial. HeartWare International Inc. announced this week that the FDA has granted conditional approval to begin enrollment in an Investigational Device Exemption (IDE) destination therapy clinical study for the HeartWare Ventricular Assist System.
Designed to enroll up to 450 patients at 50 U.S. hospitals, the non-inferiority study is a randomized, controlled, unblinded, multicenter clinical trial to evaluate the use of the HeartWare Ventricular Assist System as a destination therapy in advanced heart failure patients. The study population will be selected from patients with end stage heart failure who have not responded to standard medical management and who are ineligible for cardiac transplantation.
The primary endpoint of the trial is stroke-free survival at two years, defined as alive on the originally implanted device, transplanted or explanted due to patient recovery. Secondary endpoints include adverse events such as bleeding and infection, as well as functional status, hospitalization, assessment of neurocognitive function and patient quality of life.
There are three minor open questions raised by the FDA, which are the “conditions” to approval. Patient enrollment for the study can commence immediately, subject to Institutional Review Board (IRB) approvals at trial centers.


 January 05, 2026
January 05, 2026 









