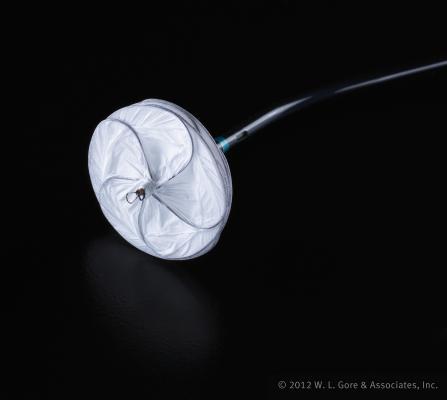
October 22, 2012 — W. L. Gore & Associates announced that the U.S. Food and Drug Administration (FDA) has approved the use of the new Gore septal occluder for inclusion in the Gore REDUCE clinical study. Gore is introducing this latest technology in its Gore REDUCE clinical study for prevention of recurrent stroke in patent foramen ovale (PFO) patients. The study is designed to demonstrate that PFO closure with the Gore device plus antiplatelet medical management reduces the risk of recurrent stroke or imaging-confirmed transient ischemic attack (TIA) when compared to antiplatelet medical management alone. The prospective, randomized, multicenter, multinational trial includes up to 80 investigational sites in the United States, Europe and Canada.
The new Gore device received CE mark in June 2011 for the indication of PFO and atrial septal defect (ASD) closure. Dr. Lars Sondergaard, Nordic Region Cardiology principal investigator for the Gore REDUCE clinical study, performed the first European procedures using the Gore device. “We have seen great success with the GORE septal occluder since we completed the first implant. The device provides ease of deployment and confidence through design for the patient’s well-being,” said Sondergaard.
Leveraging more than 13 years of clinical experience with septal occluders, the Gore septal occluder is a next-generation device that successfully integrates innovative material and design to yield a treatment option whose discs are intended to conform to the anatomy of the individual patient. The Gore device is comprised of a five-wire support frame covered with a thin ePTFE, patch-like material. The soft, strong and conformable membrane is intended to improve closure performance, providing an open microstructure to encourage fast controlled tissue ingrowth. This structure is designed to help enhance the safety of the procedure, one of several characteristics that will be investigated in the Gore REDUCE clinical study.
For more information: www.goremedical.com


 June 20, 2024
June 20, 2024 









