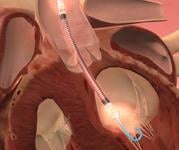
December 6, 2010 – The U.S. Food and Drug Administration (FDA) has given conditional approval for a trial that will investigate the role of a device in reducing infarct size in patients with ST-elevation myocardial infarction (STEMI). The MINI-AMI trial will use the Impella 2.5, from Abiomed, for 24 hours following primary percutaneous intervention (PCI).
“This pilot study will determine whether the Impella 2.5 can be the first device to actually shrink infarct size,” said principle investigator Jeffrey Moses, M.D., New York-Presbyterian Hospital and Columbia University Medical Center. “Today, heart attack survivors can suffer considerable subsequent disability from damaged heart muscle. The intent of this study is to investigate the unique unloading capabilities of Impella 2.5 and its effect in potentially reducing infarct size and improving quality of life for patients.”
“According to the AHA, within five years after surviving your first heart attack, 33 percent of men and 43 percent of women will die,” said Michael R. Minogue, chairman, president and CEO, Abiomed. “This will be the first Impella study to evaluate the potential therapeutic benefits of unloading and reducing the oxygen demand on the heart muscle. We are excited to explore more clinical benefits of Impella with this new, hemodynamically stable patient population.”
The primary endpoint of the study will be a cardiac MRI-assessed ratio of the final infarct area to the total area of myocardium that was at risk. This ratio will be measured at three to five days after treatment and then again at 90 days. A total of 50 patients at five hospital sites will be randomized to Impella 2.5 support or the institution’s standard of care with no circulatory support post-PCI. The study is based on the hypothesis that the device’s ability to directly unload the left ventricle will reduce overall infarct area relative to the total area at risk.
A recent study, which serves as a reference for the MINI-AMI patient population, demonstrated the following:
• Acute heart failure (AHF) during hospitalization occurred in 11.1 percent of patients in this study;
• 81.8 percent of these AHF patients died in the first 30 days;
• in some patients, even timely and successful PCI cannot prevent extensive myocardial damage;
• patients who developed AHF had a lower ejection fraction.
There was no significant difference between groups regarding the duration of symptoms or door-to-needle time.
The study was published in the Journal of Invasive Cardiology. It concluded that even after successful PCI, the occurrence of AHF during hospitalization remains an independent predictor of 30-day and one-year mortality.
For more information: www.abiomed.com


 January 05, 2026
January 05, 2026 









