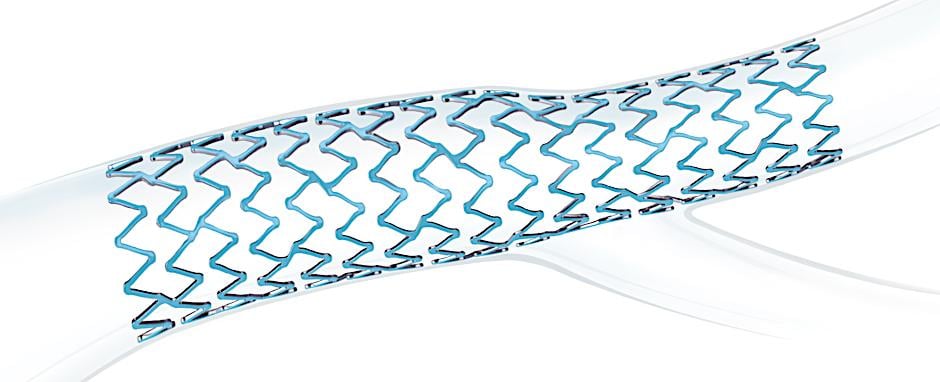
January 26, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific's Synergy Megatron Drug-Eluting Stent (DES) system. The company said it is the first platform that is purpose-built for large, proximal vessels.
Interventional cardiologists voiced an unmet need for a DES designed to treat large, proximal vessels – those that are closest to the aorta. These vessels can present unique challenges for cardiologists and Boston Scientific said the Synergy Megatron provides best-in-class radial and axial strength to maintain stent integrity. The stent also allows the largest over-expansion range on the market, which helps the device fit within tapered vessels.
The stent is designed for higher strength applications in proximal, fibrotic and calcified lesions. With its ability to be over-expanded, it can accommodate wider diameter mismatches as the vessel tapers, helping maximize lumen gain.
It also is built with a proprietary platinum chromium alloy, which is highly visible on angiography, which can help with accurate stent placement.
The stent is available in 3.5 - 5 mm diameters.
For more information: https://www.bostonscientific.com/en-US/products/stents--coronary/synergy-megatron.html


 November 14, 2025
November 14, 2025 









