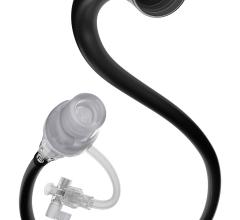
April 9, 2007 — Possis Medical Inc. has announced that the FDA has approved its AngioJet Spiroflex VG rapid exchange for blood clot (thrombus) removal in coronary conduits. Introduced in July 2006 for removing thrombus in larger peripheral arteries, today's new FDA approval allows the Spiroflex VG catheter to be marketed for use in saphenous vein bypass grafts in the heart and larger native coronary vessels.
Possis' Spiroflex VG and Spiroflex catheters are the company's most flexible and maneuverable rapid exchange catheters. The Spiroflex VG catheter provides more power than the Spiroflex catheter and is specifically designed for thrombus removal in larger peripheral and coronary vessels, while the Spiroflex catheter is uniquely suited for smaller coronary and peripheral vessel use. Both Spiroflex VG and Spiroflex catheters are now marketed for coronary and peripheral thrombus removal.
For more information visit www.possis.com


 January 19, 2026
January 19, 2026