Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston Scientific will acquire Penumbra in a cash and stock transaction.i
"Penumbra is a well-established company with an experienced, high-performing team and this acquisition offers Boston Scientific an opportunity to enter new, fast-growing segments within the vascular space," said Mike Mahoney, chairman and chief executive officer, Boston Scientific. "I'm thrilled to combine the talents and shared values of our teams – including welcoming Penumbra's chairman and chief executive officer, Adam Elsesser, to our board of directors upon close. The addition of Penumbra can expand access for these novel technologies to more patients and customers around the world, further enhancing our revenue and margins over time with proven offerings that have a history of growth and innovation."
Cardiovascular diseases are the leading cause of death globallyii and include disorders of the heart and blood vessels that can restrict blood flow and increase the risk of clots throughout the body. To address the escalating prevalence of these complex diseases, Penumbra has developed a comprehensive portfolio that includes differentiated devices to treat conditions such as pulmonary embolism, stroke, deep vein thrombosis, acute limb ischemia, heart attack and aneurysms.
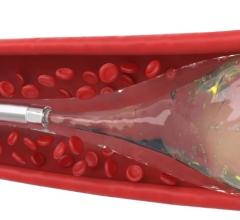
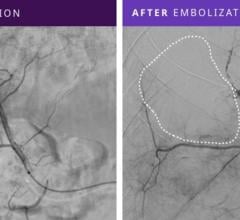
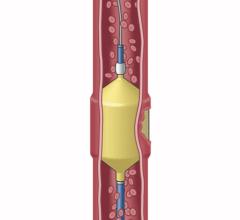
Penumbra offers innovative mechanical thrombectomy products for use in peripheral vascular procedures to remove blood clots causing blockages in arterial, venous and pulmonary vessels, including the Lightning Bolt and Lightning Flash computer assisted vacuum thrombectomy (CAVT) systems. The company's vascular portfolio also includes a minimally invasive peripheral embolization system, which is designed to stop blood flow to control hemorrhaging and bleeding or to close blood vessels.
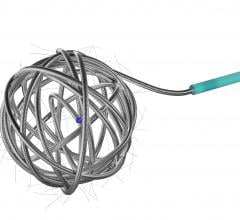
Neurovascular offerings from Penumbra currently include differentiated solutions for access, stroke revascularization and neuro embolization. The company is continuing to innovate in these areas and add meaningful clinical evidence to support expanded access for more patients worldwide.
"Our decades-long development of therapies for challenging medical conditions has focused on deep innovation for complex diseases so that we can offer physicians novel solutions to transform patient care," said Adam Elsesser. "I am grateful for the amazing people who have contributed to this work and look forward to uniting our efforts and shared values as we come together with Boston Scientific."
The transaction is expected to be completed in 2026, subject to receipt of Penumbra's stockholder approval and the satisfaction of other customary closing conditions.


 June 05, 2025
June 05, 2025