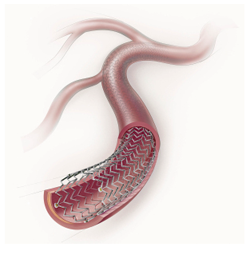
September 13, 2011 – An U.S. Food and Drug Administration’s (FDA) will discuss recommendations for Cook Medical Zilver PTX self-expanding drug-eluting peripheral stent at its Oct. 13, 2011, meeting in Gaithersburg, Md. The Circulatory System Devices Panel of the Medical Devices Advisory Committee will provide advice and recommendations to the agency on FDA's regulatory issues.
The committee will discuss, make recommendations and vote on information related to the premarket approval application (PMA) for the Cook Zilver PTX. The self-expanding nitinol stent is coated on its outer surface with the cytotoxic drug paclitaxel without any polymer, binder or excipient at a dose density of 3 micrograms/square millimeter. It is available in diameters ranging from 5 to 10 mm and lengths of 20 to 80 mm and are pre-loaded onto 6 or 7 French (diameter of 2 or 2.3 mm) delivery systems. Upon deployment, the Zilver PTX expands to establish and maintain patency in the stented region.
The proposed indications for use are treatment of de novo or restenotic symptomatic vascular disease of the above-the-knee femoropopliteal arteries having reference vessel diameter from 4 to 9 mm and total lesion lengths per patient of 280 mm.
The FDA is not bound to recommendations made by the panel, but usually follows its recommendations. If cleared by the FDA, the Zilver PTX would become the first self-expanding drug-eluting stent, the first drug-eluting stent for peripheral leg vessels and the first drug-eluting stent that does not use a polymer to carry or control the elution of the drug in the United States.
For more information: www.fda.gov/AdvisoryCommittees/Calendar/default.html


 July 02, 2024
July 02, 2024 









