September 19, 2022 — A first-in-human (FIH) study using the ModulHeart device (Puzzle Medical Devices Inc.) has demonstrated significant improvement in cardiac output, left ventricular end diastolic pressure, and urine output in patients with heart failure or undergoing high-risk percutaneous coronary intervention (PCI). The findings were presented today during the 34th Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium and simultaneously published in JSCAI, the official journal of the Society for Cardiovascular Angiography & Interventions (SCAI).
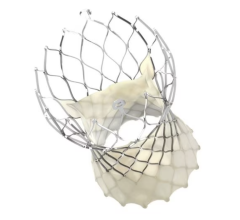
There are limited percutaneous hemodynamic support options for patients with heart failure or undergoing high-risk PCI. Current approved devices have limitations that lead to an increase in mortality, morbidity, and higher costs. The ModulHeart device is a novel modular pump implanted percutaneously, providing hemodynamic support through three endovascular pumps which are inserted in series and assembled in parallel into a dedicated self-expandable anchor in the descending aorta.
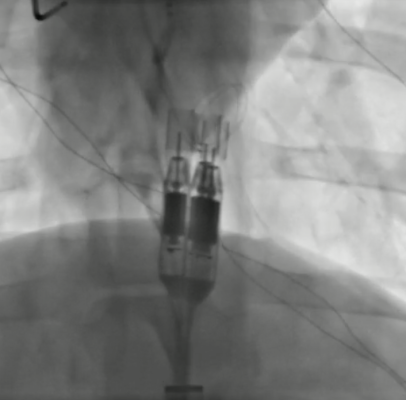
In June 2022, four patients were enrolled in the FIH study and underwent high-risk PCI with ModulHeart implanted via transfemoral approach. Mean delivery time was 8 minutes, support time was 49 minutes, and pump removal time was 7 minutes. Under ModulHeart support, cardiac index increased by 25%, central venous pressure decreased by 37%, and left ventricular end diastolic pressure decreased by 78%. Urine output increased by ~9-fold after 15 minutes of support. There was no device malfunction, procedural or device-related adverse events and all patients were alive at 30 days post procedure.
“The first-in-human experience using the ModulHeart device was extremely positive, showing great cardiorenal support and pump function,” said Dr. Philippe Généreux, interventional cardiologist at the Gagnon Cardiovascular Institute at Morristown Medical Center in New Jersey, and senior author of the study. “The device was easy to deliver and assemble. Once in place, it provided substantial hemodynamic benefits. I am looking forward to the upcoming studies to reduce congestion in patients with acute decompensated heart failure.”
The current study was performed among patients undergoing high-risk PCI with some degree of left ventricular dysfunction. The researchers state that future studies will target patients with acute and chronic decompensated heart failure with more profound reduced ejection fraction and requiring hemodynamic support and will also explore longer use of the device during the procedure.
For more information: www.scai.org


 May 05, 2025
May 05, 2025