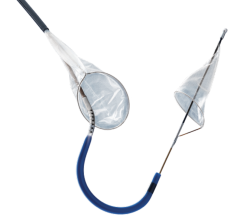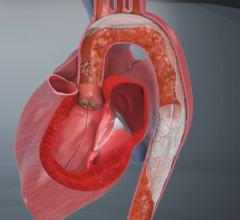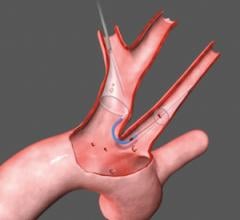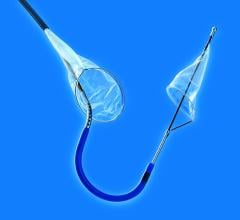
March 10, 2009 - W. L. Gore & Associates today said the first clinical use of the GORE Flow Reversal System for neuroprotection during carotid artery stenting (CAS) was performed by Daniel McCormick, M.D. and Sheldon Goldberg, M.D., interventional cardiologists at Hahnemann University Hospital, Philadelphia.
Gore obtained 510(k) clearance from the FDA for the system in February. The GORE Flow Reversal System is a new technology, which minimizes the risk of emboli reaching the brain during critical stages of CAS, thereby expanding treatment options for broad patient populations with carotid artery disease.
“We are pleased to be the first site to perform a procedure using the commercially available GORE Flow Reversal System,” said Dr. McCormick. “I believe this new development in carotid artery stenting is a promising advancement in the treatment of carotid disease.”
The first patient was recently symptomatic with an extreme aortic arch and a string sign lesion in the internal carotid artery. The lesion was successfully treated under neuroprotection using the GORE Flow Reversal System. The patient was discharged from the hospital and is doing well.
“I was impressed that the GORE Flow Reversal System negotiated the Type III aortic arch with ease,” stated Dr. McCormick. “Treating this extremely tight stenosis and challenging arch anatomy under flow reversal provided me with a level of performance and confidence that would have been improbable with other embolic protection devices.”
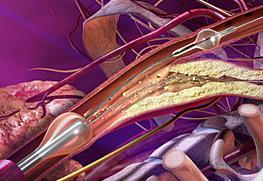
One of the major challenges associated with CAS is the risk of periprocedural embolic events that could cause a stroke. The GORE Flow Reversal System establishes neuroprotection by reversing blood flow at the treatment site prior to crossing the lesion. The company said flow reversal is achieved by selectively occluding common carotid and external carotid artery blood flow. By establishing an arterio-venous shunt, blood from collateral vessels via the Circle of Willis is redirected to the lower pressure venous return. Macro and micro emboli are continuously directed away from the brain during flow reversal.
Prior to the procedure, Dr. McCormick attended a GORE Medical Mastery Series Flow Reversal Course to gain familiarity with the system. This course allows clinicians to perform simulated carotid artery procedures using the product and educates physicians on how flow reversal makes the promise of carotid artery stenting possible.
Gore is conducting other studies in the area of stroke intervention including the Gore REDUCE Clinical Study evaluating the GORE HELEX Septal Occluder for patent foramen ovale closure and stroke prevention, and the Gore EMBOLDEN Clinical Study evaluating the GORE Embolic Filter in CAS.
*REDUCE: GORE HELEX Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients with Patent Foramen Ovale (PFO)
For more information: www.goremedical.com


 April 25, 2023
April 25, 2023