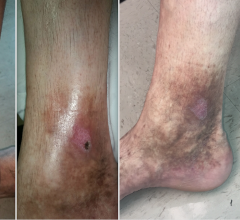
August 18, 2014 — Polidocanol injectable foam (Varithena), the only U.S. Food and Drug Administration (FDA)-approved foam for the treatment of incompetent great saphenous veins (GSV), accessory saphenous veins and visible varicosities of the GSV system both above and below the knee.
Polidocanol injectable foam improves symptoms related to or caused by varicose veins, and the appearance of varicose veins, and is proven to reduce the five symptoms patients consider most important — heaviness, achiness, swelling, throbbing, itching (HASTI symptoms).
Marlin Schul, M.D., M.B.A., of the Lafayette Regional Vein & Laser Center in Indiana, who conducted the first Polidocanol injectable foam procedure, said, "I am proud to now be able to offer Varithena as a new treatment option for my patients with varicose veins. Varithena is a convenient, minimally invasive treatment and patients can return to normal activities shortly after treatment."
Paul McCubbin, head of Varithenaat BTG, commented, "Varithena is the first and only FDA-approved comprehensive treatment that improves the symptoms and appearance of varicose veins. We are delighted that this clinically proven product is now commercially available to qualified physicians."
Polidocanol injectable foam is a uniform, low-nitrogen, polidocanol microfoam, dispensed from a proprietary canister device. The physician injects a small amount of polidocanol injectable foam into the malfunctioning vein through a small tube (catheter) or a needle. It displaces the blood from the vein to reach and treat the vein wall; the diseased vein collapses and blood flow is diverted to healthy veins nearby.
For more information: www.varithena.com


 September 10, 2025
September 10, 2025