November 10, 2017 — GE Healthcare and Dutch-based cardiovascular imaging software provider Medis announced at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) annual conference a new collaboration agreement to increase the clinical availability and adoption of Medis Suite QAngio XA 3D, a proprietary image-based fractional flow reserve (FFR) technology.
Coronary artery disease (CAD) develops when the coronary arteries narrow, reducing blood flow to the heart, resulting in angina (chest pain), myocardial infarction (heart attack) or death. When diagnosing suspected CAD, there is no room for doubt.
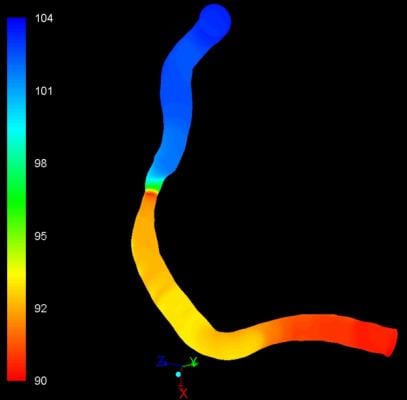
“Image-based QFR [quantitative flow ratio] can quickly and efficiently help a clinician non-invasively determine whether or not they need to perform angioplasty or stenting in five minutes or less,” said Pr. Hans Reiber, Ph.D., president and CEO of Medis. “This is significantly quicker than traditional wire-based FFR procedures that take about 20 minutes, are invasive and require the use of a hyperemic drug, making the procedure very demanding on a patient.”
QFR is based on angiographic images and direct coronary flow estimation and allows for fast in-procedure results. This software solution is designed to be X-ray vendor-independent and to be used on both biplane and monoplane X-ray systems. Image selection is facilitated through an angiographic acquisition guide and the total analysis time is typically around 5 minutes including image frame selection.
By using QFR, clinicians can significantly reduce stent overuse and associated risks, better determine the correct stent length and help establish optimal viewing angles for stent positioning. The results are better for the patient and the clinician, according to Medis.
For more information: www.gehealthcare.com, www.medis.nl


 October 24, 2025
October 24, 2025