Compared to the current devices used to treat sudden cardiac arrest, the new internal defibrillator has less risk of infection, no clots forming in blood vessels, no lead perforation through the heart wall and does not puncture the lining of the lung. Also, the lead is not subject to normal wear and tear of heart movement, as it is just under the skin — not inside the heart.
"This device should be considered a first-line treatment for patients at risk for sudden cardiac arrest who do not require the regular stimulation of a pacemaker," said
Arfaat Khan, M.D., cardiologist, Henry Ford. "Patients with an increased risk of infection will benefit the most, such as those with diabetes or on dialysis, as well as young patients that may need to have leads replaced as they wear out over time.”
"It has the potential to become the new standard of care," Khan added.
Henry Ford is the first hospital in Michigan to implant this device, the only subcutaneous defibrillator to treat patients at risk for sudden cardiac arrest.
Traditional internal cardiac defibrillators have proven to be effective in the treatment of sudden cardiac death. Each year, approximately 200,000 of them are implanted in American patients who are at risk of sudden cardiac arrest.
Eighty-six-year-old Gale Irwin of Taylor is a retired letter carrier who walks regularly. But he was getting short of breath in the spring, and his doctor discovered he had had a small heart attack. At a follow-up exam after having a triple heart bypass, his doctor did not feel that his heart was functioning well enough, although he did not need a pacemaker. Irwin became the first person in Michigan to receive the new device and was discharged from the hospital the next day.
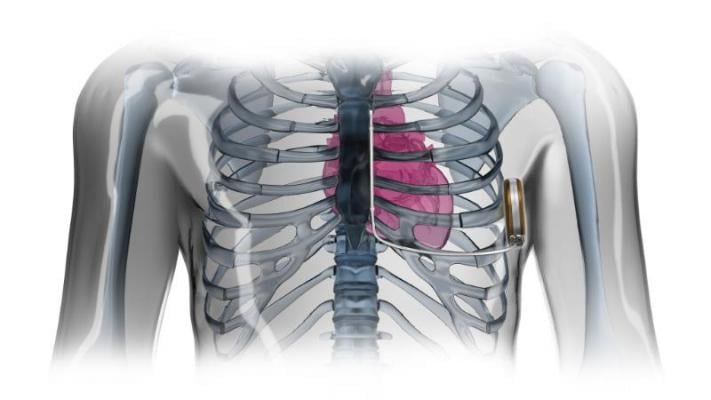
The new defibrillator is designed to provide the same protection from sudden cardiac arrest as traditional implantable defibrillators, but it does not require an electrical lead in the heart. Rather, the lead is implanted just under the skin, along the bottom of the rib cage and breastbone, and does not touch the heart. Since it does not travel through a vein into the heart, a physician can implant the device without going through blood vessels and without X-ray fluoroscopy.
The device has two main components: the pulse generator, which powers the system, monitors heart activity and delivers a shock if needed; and the electrode, which enables the device to sense the cardiac rhythm and serves as a pathway for the shock delivery. The generator is placed at the side of the chest and the electrode beside the breastbone.
Boston Scientific’s S-ICD system is U.S. Food and Drug Administration (FDA)-approved.
For more information: www.henryfordhospital.com, www.bostonscientific.com