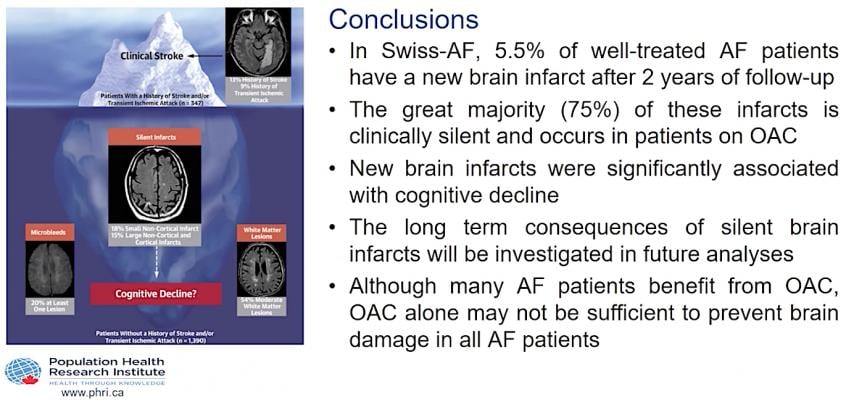
May 8, 2020 – A new clinical study found that patients with atrial fibrillation (AF) experienced a high incidence of clinically silent brain infarcts, despite taking oral anticoagulants for stroke prevention. Silent brain infarcts were significantly associated withcognitive decline. The results of the Swiss Atrial Fibrillation Cohort (Swiss-AF) trial were presented as a late-breaking clinical trial as part of Heart Rhythm Society (HRS) 2020 Science virtual meeting.
AF patients have a high risk of cognitive dysfunction and dementia, in part due to their high risk of ischemic stroke. To help mitigate the risk of an ischemic stroke, many patients with AF are prescribed oral anticoagulation. Clinically silent brain infarcts can be hard to detect in patients and have a similar impact on cognitive decline as an actual stroke. The incidence of silent brain infarcts in anticoagulated patients and whether they impact cognitive function is currently unknown. The Swiss-AF study sought to better understand the role clinically silent brain infarcts play in cognitive decline in AF patients.
The multicenter observational cohort study enrolled 1,227 patients aged ≥65 with previously documented AF. The mean age at baseline was 71.5 ± 8.4 years, and 26 percent were women. Patients underwent brain magnetic resonance imaging (MRI) at baseline and after two years of follow-up. Brain infarcts were quantified in a central core laboratory. New infarcts were defined as lesions present on the follow-up MRI scan but not at baseline, while clinically silent infarcts were defined as new infarcts in patients without a clinical stroke between the two MRI studies.
In the Swiss-AF study, 84 percent of study participants received oral anticoagulation. Nonetheless, 5.5 percent of the participants had a new brain infarct after two years of follow-up. Of the 68 patients with new brain infarcts, 58 (85 percent) had a clinically silent event, 59 (87) were taking oral anticoagulation during the follow-up period, and 51 (75 percent) had a clinically silent brain infarct while taking oral anticoagulation. Patients with new brain infarcts had a stronger decline in cognitive function than patients without new brain infarcts.
“When it comes to our understanding of the link between clinical stroke and AF, what we have known so far is only the tip of the iceberg. This clinical study set out to understand why AF patients suffer from higher rates of cognitive decline even without experiencing a stroke, and provide the clinical field a better understanding of the impact silent brain lesions have on cognitive decline,” said lead author David Conen, M.D., MPH, Associate Professor in the Division of Cardiology and Department of Medicine at McMaster University. “Although many AF patients benefit from oral anticoagulants, additional treatment might need to be considered in some patients to prevent silent brain infarcts. Our hope is that these results will help physicians advance treatment options for AF management among their patients and help mitigate the risk of cognitive decline, dementia and stroke.”
The authors of this study call for additional trials to further study the long-term consequences and impacts of silent brain infarcts in AF patients. Through additional research, the authors also hope to better understand which risk factors are associated with new silent brain infarcts.
Find links to all the Heart Rhythm Society 2020 Late-Breaking Clinical Trials in Electrophysiology


 August 28, 2023
August 28, 2023 









