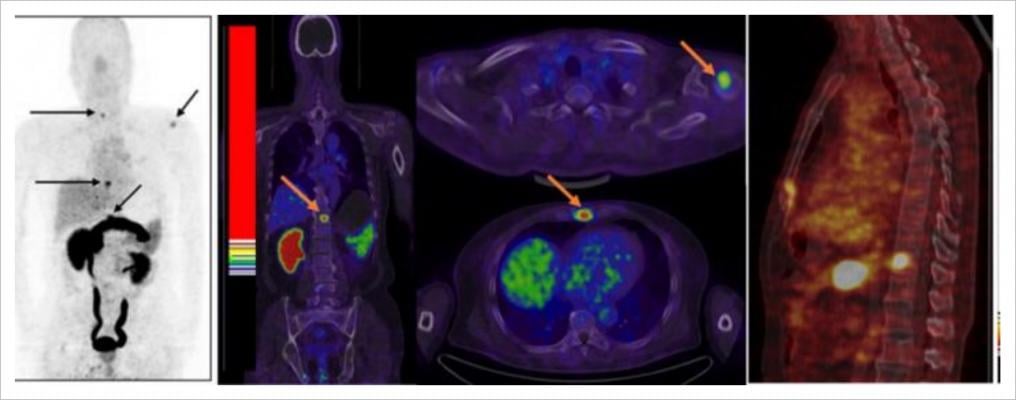
July 13, 2021 — In a recent blog, the American Society of Nuclear Cardiology (ASNC) reported that Humana, one of the largest commercial payers in the U.S., has reversed its Fusion policy, which restricted payment for certain PET/CT exams on the grounds that they were “experimental/investigational.” The decision follows meetings where ASNC President Randall C. Thompson, M.D., FASNC, and CPT Editorial Panel Advisor Friederike Keating, M.D., presented robust published data supporting PET/CT's clinical value to Humana's medical director. You can read the ASNC’s comments here.
Humana’s new draft Fusion policy outlines coverage determinations for myocardial assessment and the use of PET/CT and SPECT/CT for certain neurologic indications. ASNC has submitted additional feedback on the draft policy.
“We’re pleased to see Humana’s initial draft Fusion policy and hope that our further feedback to Humana will be incorporated in the final draft policy and will recognize the clinical value of PET/CT for MPI,” said Keating.
For more information: www.asnc.org


 January 05, 2023
January 05, 2023