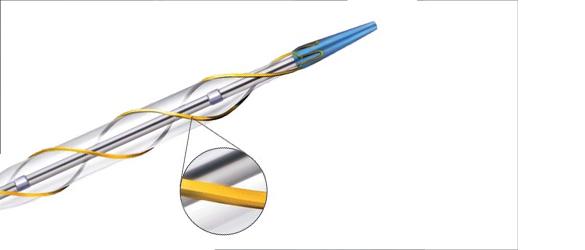
April 28, 2010 – Deviating from its focus on intravascular imaging systems and fractional flow reserve (FFR) catheters, Volcano Corp. said yesterday it entered into a Japanese distribution agreement with AngioScore for its AngioSculpt PTCA scoring balloon catheter. This is the first of what Volcano believes will be many products distributed by Volcano Japan.
This relationship combines Volcano’s intravascular ultrasound (IVUS) visualization with the AngioSculpt to provide precision-guided therapy. The use of IVUS has already taken hold in Japan, where the technique is used in more than 70 percent of all percutaneous coronary interventions (PCI).
Over the past 18 months, Volcano has built a direct sales force in Japan. The exclusive, five-year distribution agreement became effective April 1. Volcano will distribute the entire line of AngioSculpt catheters and any new, modified or next-generation products.
Volcano is also developing is own angioplasty balloon, which is integrated with an IVUS catheter. The company hopes to launch the product by the end of 2010 and said it will be the first of several interventional therapy/visualization tools it plans to release.
For more information: www.volcanocorp.com, www.angioscore.com


 June 13, 2024
June 13, 2024 









