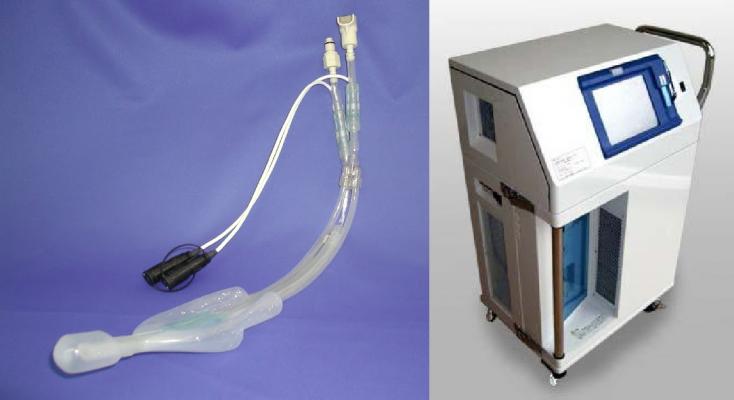
December 11, 2014 — Researchers at Okayama University in collaboration with several medical centers in Japan have demonstrated the safety and efficacy of a hypothermal treatment – pharyngeal cooling – for cardiac arrest patients.
Cooling the brain is known to prevent neurological problems in patients recovering from cardiac arrest. However, most of the current methods for therapeutic hypothermia may not be initiated before return of spontaneous circulation.
Researchers at Okayama University investigated a method for cooling the area at the top of the throat — the pharynx — because the arteries that supply the head with oxygenated blood run nearby. Cooling this area should be a good approach to cooling the brain but so far there have been no complete studies to determine whether pharyngeal cooling could be administered effectively or whether it may lead to other adverse side effects.
The researchers, alongside people working in emergency and critical care clinics, set up a trial for 108 cardiac arrest patients. The medical staff administered treatments with or without pharyngeal cooling to patients at random, and subsequently recorded success rates of resuscitation and physiological conditions, including temperature both at the body core and in the head near the ear (tympanic temperature), mechanical or temperature damage to the pharynx, inflammation and blood platelet levels.
The results of the trial indicated effective cooling of tympanic temperatures, with no observed adverse side effects. In addition, incidences of inflammation and blood-clotting disorders were reduced in patients receiving pharyngeal cooling. As the researchers report, “In conclusion, it appears that the initiation of pharyngeal cooling is safe and feasible before and shortly after recovery of spontaneous circulation in the emergency room.”
For more information: www.okayama-u.ac.jp


 January 05, 2026
January 05, 2026 









