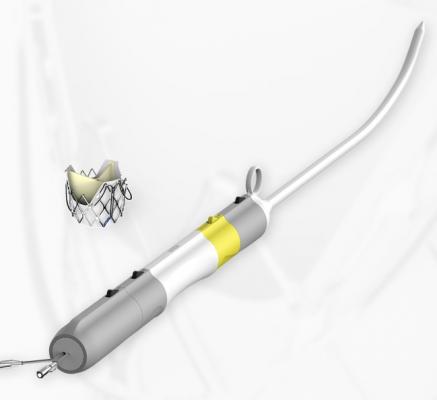
January 7, 2019 — JC Medical announced the successful treatment of the first U.S. patient with the company’s transfemoral transcatheter aortic valve replacement (TAVR) device, the J-Valve TF System. The patient was treated at The Christ Hospital – Cincinnati, Ohio by Dean Kereiakes, M.D., FACC, FSCAI, medical co-director of the Lindner Research Center, working with Joseph Choo, M.D., and Geoffrey Answini, M.D.
The U.S. Food and Drug Administration (FDA) approved the use of the J-Valve for patients with aortic regurgitation through the agency’s expanded access (“compassionate use”) regulatory pathway.
The investigational J-Valve TF system is intended as a new catheter-based option for the treatment of patients who suffer from aortic regurgitation. Failing aortic valves make up a significant portion of patients with heart failure, a major public health problem with a prevalence of more than 1.5 million people in the U.S. and more than 18 million people worldwide. Aortic regurgitation is the primary indication for more than 20 percent of surgical heart valve replacements, but there are no transcatheter aortic valves that are approved in the U.S. or Europe to treat aortic regurgitation patients too sick to undergo open surgical repair.
“The J-Valve TF system has specific attributes that differentiate it from all other currently available TAVR systems, which I believe will enhance the safety and efficacy of TAVR for the indications of aortic valvular insufficiency (regurgitation) and possibly valve-in valve replacement of failing surgically implanted bioprosthetic valves,” said Kereiakes. “J-Valve was clearly the best treatment option for our patient.”
JC Medical’s J-Valve System is designed to restore normal blood flow out of the heart and into the body, which may improve symptoms of heart failure such as shortness of breath, fatigue and chest pain. The J-Valve features a proprietary anchor mechanism that is flexibly linked to a self-expanding stent frame to attach to the failing native heart valve. The J-Valve does not require calcification of the native valve for fixation.
The world’s first J-Valve TF implant was performed earlier this year by John Webb, M.D., and Jian Ye, M.D., MSc, FRCSC at St. Paul’s Hospital in Vancouver, Canada. This case was recently published online ahead of print in EuroIntervention.1
The company plans to initiate a U.S. clinical trial of the J-Valve in 2019.
For more information: www.j-valve.com
Reference


 November 14, 2025
November 14, 2025 









