September 28, 2018 — The first randomized study to compare general versus local anesthesia during transcatheter aortic valve replacement (TAVR) in patients with intermediate to high surgical risk found local anesthesia to be both safe and effective. In addition, the study found that a current generation balloon-expandable valve had similar outcomes to a current generation self-expanding one.
Findings from the SOLVE-TAVI trial were reported at the 30th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, Sept. 21-25 in San Diego.
TAVR is increasingly performed in symptomatic patients with severe aortic stenosis at high to intermediate surgical risk. Based on registry data, approximately half of the procedures are performed with general anesthesia and the other half with local anesthesia with possible conscious sedation. The registry data also suggests lower mortality, lower morbidity, shorter intensive care unit (ICU) and hospital stay, and shorter procedure times with local anesthesia. However, there is a lack of adequately powered randomized trials comparing the two types of anesthesia in TAVR.
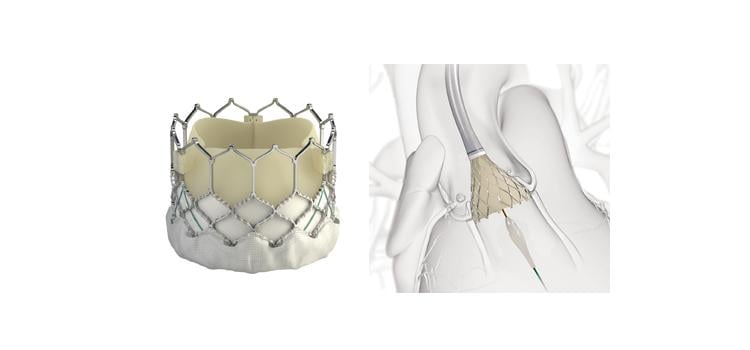
In this randomized, prospective, multicenter trial, 447 patients with severe symptomatic aortic stenosis and intermediate to high surgical risk were randomized in a 2 by 2 factorial design. Patients were randomized to either general or local anesthesia with conscious sedation, and also to the balloon-expandable Sapien 3 valve or the self-expanding CoreValve Evolut R.
The primary endpoint for the valve strategy was all-cause mortality, stroke, moderate or severe prosthetic valve regurgitation, and permanent pacemaker implantation at 30 days. The endpoint occurred in 27.2 percent of the Evolut R group compared to 26.1 percent of the Sapien 3 group (Rate difference -1.14; 90 percent CI: -8.15 – 5.87; Pequivalence=0.02). Examining individual components of the primary endpoint, the rate of relevant valve regurgitation and permanent pacemaker rates were similar with both devices; however, there was a rate of stroke with the balloon expandable valve.
The primary efficacy endpoint for the anesthesia comparison was powered for equivalence and consisted of the composite of all-cause mortality, stroke, myocardial infarction, infection requiring antibiotic treatment, and acute kidney injury at 30 days. The primary endpoint occurred in 27 percent of the local anesthesia group compared to 25.5 percent of the general anesthesia group (Rate difference -1.52; 90% CI: -8.47 – 5.42; Pequivalence=0.02). General anesthesia was associated with a higher rate of catecholamine use but did not affect procedure times, valve-related outcome or clinical outcomes.
“The SOLVE-TAVI trial is the first adequately powered randomized trial comparing local versus general anesthesia in patients with symptomatic aortic valve stenosis undergoing TAVR,” said Holger Thiele, M.D., director of the Heart Center Leipzig - University Hospital in Leipzig, Germany. “Results indicate that local anesthesia is both safe and effective and may be a good option for those patients undergoing TAVR with an intermediate or high surgical risk.”
The SOLVE-TAVI trial was funded by German Heart Research Foundation. Thiele has no conflicts of interest.
For more information: www.crf.org


 January 05, 2026
January 05, 2026 









