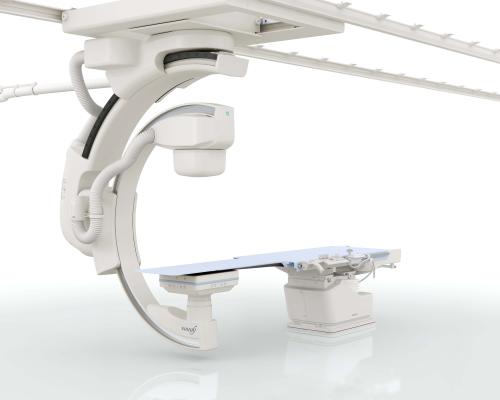
April 18, 2017 — Patients at Terrebonne General Medical Center (TGMC) in Houma, La., now have access to safe, high-quality interventional cardiac exams with the installation of Toshiba Medical’s Infinix-i Sky +. TGMC, a nationally recognized healthcare organization and home to one of the most advanced vascular interventional practices in the country, is the first healthcare provider in the United States to install the Infinix-i Sky +. The center is leveraging the system’s flexibility and high-quality imaging to perform a wide array of procedures, enabling clinicians to provide outstanding care to more patients.
“Innovative imaging technology like the Infinix-i Sky + helps us provide the best possible patient care by making the types of procedures we perform safer,” said Peter Fail, M.D., director of cardiac catheterization laboratories and interventional research, Terrebonne General Medical Center and Cardiovascular Institute of the South. “The system’s dramatic improvements in visualization, including its large flat panel detector and wide display screen, streamline workflow and help us treat patients quickly and safely.”
TGMC is using the Infinix-i Sky + for a variety of interventional procedures including transcatheter aortic valve replacement (TAVR), peripheral vascular surgeries, appendage applications and hybrid coronary bypass/stenting procedures. The ceiling-mounted system features a double sliding C-arm that allows the C-arm to be positioned in more ways to help doctors increase coverage, speed and patient access. The system’s 12 x 16-inch flat panel can be positioned above or below the patient, offering physicians increased flexibility for procedures that require additional space, and its low contrast imaging capabilities.
The Infinix-i Sky + also incorporates an extensive set of automated and user-selectable dose management tools, designed to help clinicians reduce X-ray exposure. This includes technology like the Dose Tracking System that estimates peak skin dose exposure in real time.
For more information: www.medical.toshiba.com


 October 24, 2025
October 24, 2025 









