January 20, 2021 — The U.S. Centers for Medicare and Medicaid Services (CMS) revised its National Coverage Determination (NCD) to expand coverage for the Abbott MitraClip to include patients with secondary (or functional) mitral regurgitation (MR) resulting from heart failure.
The procedure is known as transcatheter edge-to-edge repair (TEER), but is also referred to as transcatheter mitral valve repair (TMVR).
The CMS decision significantly increases the number of people eligible for insurance coverage for mitral valve repair with MitraClip, enabling broader access to the device. As the first TEER device approved by the U.S. Food and Drug Administration (FDA) and reimbursed by Medicare for primary mitral regurgitation, physicians have increasingly relied on the therapy to improve survival and quality of life for their patients. Today's decision improves insurance coverage for people with secondary MR who need treatment with MitraClip.
"Secondary mitral regurgitation generally impacts older individuals suffering from heart failure who rely on Medicare for their healthcare coverage," said Neil Moat, M.D., chief medical officer of Abbott's structural heart business. "CMS' decision to expand coverage for MitraClip marks a pivotal moment for people seeking a minimally invasive option that reduces mitral regurgitation and significantly improves their quality of life and chances of survival."
Following MitraClip's original approval by the FDA in late 2013, CMS provided coverage for Medicare patients with primary (degenerative) mitral regurgitation who needed treatment with MitraClip. Similarly, this revised NCD comes after the FDA's 2019 expanded indication for MitraClip to treat people with secondary MR.
MitraClip Clinical Data Continues To Improve
The decision also follows the highest MR reduction data for MitraClip reported to date. Recent 2020 data demonstrated MR reduction was consistently achieved with MitraClip in patients with either primary MR (to ≤1+ in 87.1%) or secondary MR (to ≤1+ in 90.1%) at 30 days.[1]
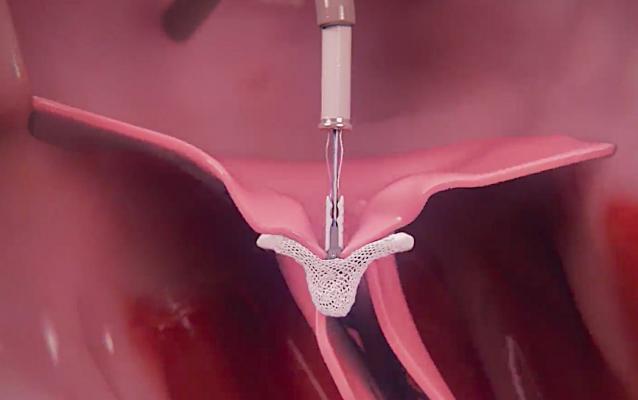
Abbott said the device is supported by more than 17 years of clinical trial data demonstrating safety and efficacy, and it is now on the fourth generation of product innovations. MitraClip is a first-of-its-kind device that clips together the mitral valve leaflets to reduce the backflow of blood and restore the heart's ability to pump oxygenated blood efficiently. The device provides symptom relief for both primary and secondary MR, with most patients being released from the hospital, on average, two days post-procedure.[2]
Heart Failure Causes Enlargement of the Heart Leading to MR
People with heart failure are at risk of developing significant secondary MR, a serious and progressive heart disease in which the two chambers of the left side of the heart become enlarged. This causes thus preventing the mitral leaflets from closing normally and allowing blood to flow backwards through the heart. This can lead to reduced quality of life, recurrent hospitalizations and decreased survival.[3,4,5] Most heart failure patients with clinically significant secondary MR are treated with medication only, but data shows that these patients can benefit from reduction in MR with MitraClip.
Abbott said this NCD revision is critically important to secondary MR patients, as most of those impacted by MR are older, with one in 10 adults age 75 or older experiencing MR,[6] and therefore relying on Medicare for their health insurance. Medicare coverage is limited to treatments, procedures and other healthcare items or services that CMS considers reasonable and necessary. National Coverage Determinations mandate coverage at the national level for all Medicare beneficiaries, including those participating in Medicare Advantage plans.
Had CMS not revised the NCD to include the new secondary MR indication, Medicare patients with the secondary form of the disease would have continued to be ineligible for coverage and required to pay out of pocket for the procedure, creating a barrier to therapy access for patients.
About the MitraClip Device
MitraClip is now approved in more than 75 countries worldwide.
The MitraClip system has been commercially available in Europe since 2008 and was approved in the U.S. in 2013 for primary MR patients. In March 2019, the FDA approved MitraClip for secondary MR patients based on results from the landmark COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) Trial,[7] which investigated MitraClip for treating secondary MR. COAPT showed a 47 percent relative reduction in hospitalizations and a 38 percent relative reduction in mortality for secondary MR patients. Both primary and secondary MR patients may benefit from MitraClip therapy based on this expanded indication for MitraClip.
For more information on MitraClip: www.MitraClip.com
For U.S. safety information on MitraClip: http://abbo.tt/MitraClipG4ISI
References:
1. Rottbauer W. D. Contemporary Clinical Outcomes with MitraClip (NTR/XTR) System: Core-lab Echo Results from +1000 Patient the Global EXPAND Study. Data presented at PCR 2020.


 November 14, 2025
November 14, 2025 









