Feb. 3, 2026 — Medtronic plc announced it will exercise its option to acquire CathWorks, a privately held medical device company, which aims to transform how coronary artery disease (CAD) is diagnosed and treated.
The intent to acquire CathWorks follows a 2022 strategic partnership with a co-promotion agreement for the CathWorks FFRangio System in the U.S., Europe and Japan, where it is commercially available.
"Medtronic is thrilled to move forward with our option to officially acquire CathWorks. Through our co-promotion agreement, we've seen how CathWorks can disrupt the traditional wire-based FFR segment and leverage the power of data and AI to deliver innovative solutions that assist physicians at every step of a patient's journey, from diagnosis to treatment," said Jason Weidman, senior vice president and president of the Coronary & Renal Denervation business, which is part of the Cardiovascular Portfolio at Medtronic. "This acquisition allows Medtronic to transform the cath lab with a technology that provides real-time data, informs individualized treatment approaches, and drives new standards of care."
Evaluating the physiological significance of coronary artery stenosis is essential to improving patient outcomes. Coronary physiology, most commonly assessed using fractional flow reserve (FFR), helps physicians identify which lesions truly cause ischemia. This enables appropriate revascularization for patients who need it, while avoiding unnecessary percutaneous coronary intervention (PCI) in those who do not. FFR is an important diagnostic tool with strong clinical evidence that demonstrates its improved clinical outcomes and economic benefits.1, 2 Despite its proven benefits, traditional wire-based FFR remains underutilized. This is largely due to its invasive nature, which requires the use of pressure wires, pharmacologic hyperemia, and measurements limited to a single transducer location within the vessel.
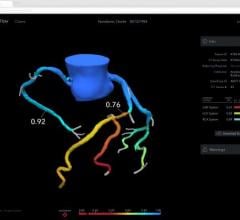
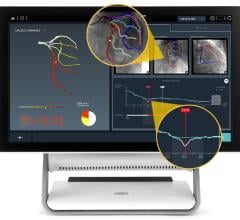
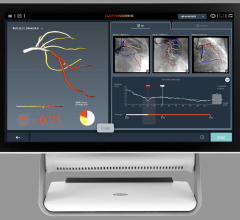
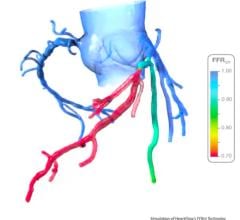
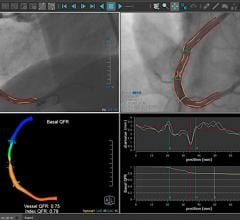
Alternatively, using a combination of artificial intelligence (AI) and advanced computational science, the CathWorks FFRangio System provides a comprehensive physiological assessment of the entire coronary tree directly from routine coronary angiograms (X-rays). Robust clinical evidence has demonstrated excellent diagnostic accuracy and promising clinical outcomes when compared with wire-based FFR.3,4
This deal is pending clearance from the United States Federal Trade Commission (FTC). This phase is expected to be completed by the end of Medtronic's fiscal year 2026, subject to applicable regulatory approvals and other customary closing conditions. At the date of the acquisition closing, CathWorks will then become a part of Medtronic. Medtronic and CathWorks will continue to operate independently until the deal is closed.
1. Bernard De Bruyne, M.D., Ph.D., Nico H.J. Pijls, M.D., Ph.D., Bindu Kalesan, M.P.H., Emanuele Barbato, M.D., Ph.D., Pim A.L. Tonino, M.D., Ph.D., Zsolt Piroth, M.D., Nikola Jagic, M.D., Sven Möbius-Winkler, M.D., Gilles Rioufol, M.D., Ph.D., Nils Witt, M.D., Ph.D., Petr Kala, M.D., Philip MacCarthy, M.D., Thomas Engström, M.D., Keith G. Oldroyd, M.D., Kreton Mavromatis, M.D., Ganesh Manoharan, M.D., Peter Verlee, M.D., Ole Frobert, M.D., Nick Curzen, B.M., Ph.D., Jane B. Johnson, R.N., B.S.N., Peter Jüni, M.D., and William F. Fearon, M.D., for the FAME 2 Trial Investigators. Fractional Flow Reserve–Guided PCI versus Medical Therapy in stable coronary disease. The New England Journal of Medicine 2012; 367:991-1001DOI: 10.1056/NEJMoa1205361
2. Fearon et al. Economic Evaluation of Fractional Flow Reserve-guided Percutaneous Coronary Intervention in Patients with Multivessel Disease. Circulation. 2010;122:2545-2550.
3. Witberg G, De Bruyne B, Fearon WF, Achenbach S, Engstrom T, Matsuo H, et al. Diagnostic performance of angiogram-derived fractional flow reserve: a pooled analysis of 5 prospective cohort studies. JACC Cardiovasc Interv. (2020) 13:488–97. 10.1016/j.jcin.2019.10.045
4. Tanigaki T, et al. Provision Trial. Data presented at PCR 2025; Paris, France


 October 30, 2024
October 30, 2024