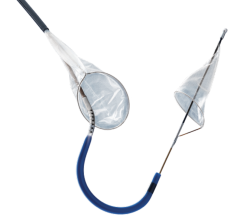
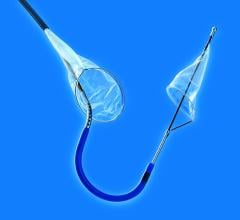
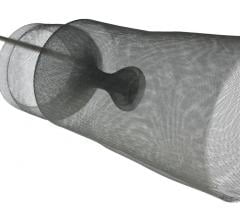
April 29, 2008 - Medtronic received CE mark approval for the Defender embolic protection filter, a device designed to get rid of emboli that can occur during a variety of stenting procedures.
Made of braided nitinol (a memory metal? that resumes its original shape upon deployment), the Defender embolic protection filter has a low profile and a peel-away delivery sheath that enables physicians to maneuver the device easily across lesions to the desired location. When opened, the filter acts as a basket, allowing sufficient blood flow while simultaneously trapping dangerous embolic debris that may become dislodged during the stenting procedure. Without this protection, embolic debris can flow into other portions of blood vessels and, in the case of patients with carotid artery disease, this can lead to a stroke, one of the world's leading causes of death and long-term disability.
The Defender filter has a 2.2 French (0.029 inch) crossing profile and an extendable 0.014 inch stainless steel core wire that is designed for both flexibility and support. Also, the device's mesh filter basket design allows the filter to fit snugly against vessel walls, even in eccentrically shaped vessels. This reduces the risk of embolization by preventing particles from migrating through gaps and into the blood stream. Another key feature of the device is its steerability and guidewire-like torque response, which measures the number of revolutions needed to turn the tip 180 degrees within the sheath. Bench testing shows the Defender filter to have improved torque response.
"Torque response is a key attribute for a filter, and Defender performed very well, with a 1-to-1 torque response between the proximal and distal tip of the wire,"? said Dierk Scheinert, M.D., director of the Park-Hospital Leipzig and head of the Department of Angiology at the University of Leipzig Heart Center. "We were impressed with the trackability of the system. One of the cases had a long lesion (2 cm) to cross, and the filter went through without any issue."
For more information: www.medtronic.com


 April 25, 2023
April 25, 2023