April 27, 2022 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall for the Medtronic Harmony delivery catheter, part of the transcatheter pulmonary valve (TPV) system, for risk of capsule break during use. Use of these devices may cause serious injuries or death, according to the FDA's statement.
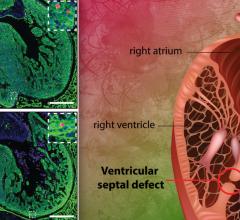
The Harmony Transcatheter pulmonary valve (TPV) system is used to treat a leaky native or surgically repaired right ventricular outflow tract (RVOT), which is the part of the heart that carries blood to the lungs. The Harmony TPV System consists of a transcatheter pulmonary valve and a delivery catheter, the Harmony Delivery Catheter, that is used to implant the replacement valve without open heart surgery.
The system is indicated for use in children and adults who have severe pulmonary regurgitation, which is when blood flows backward from the RVOT into the right lower chamber (right ventricle) of the heart, and require replacement of the pulmonary valve.
Reason for Recall
Medtronic is recalling the Harmony Delivery Catheter because it is possible that the bond holding the capsule at the end of the delivery catheter may break during a procedure to place the TPV.
A capsule bond break could cause procedure delays while the device is replaced with a new one or it may require the patient to undergo additional surgeries. Additionally, a capsule bond break while in use during a procedure could cause serious harm to the patient. Those risks include preventing blood flow and/or completely blocking (embolization or occlusion), tearing and/or splitting (perforation or dissection), or other types of damage to the patient’s blood vessels.
There have been 6 reported complaints from clinical cases, one injury, and no deaths associated with the use of these devices.
Who May be Affected
- Health care personnel who plan to implant the Harmony TPV into patients with severe pulmonary regurgitation
- People who are candidates for valve replacement using the Harmony TPV system
What to Do
On April 6, 2022, Medtronic issued an Urgent Medical Device Recall notice to implanting physicians and customers recommending immediate suspension of use for Harmony TPV’s delivery catheter.
Instructions for customers included:
- Removing all unused products from use and returning them to Medtronic.
- Pausing new clinical cases involving the Harmony TPV System.
Customers were also asked to complete a form that was enclosed with the letter to confirm receipt of the recall and to report the number of unused devices currently on hand.
The letter also specified that the recall is:
- Specific to the Harmony Delivery Catheter only, and not the Harmony TPV.
- The break has only occurred in the delivery catheter during delivery of a TPV, no actions are needed for patients who have already been successfully implanted with a Harmony TPV.
Contact Information
Customers with questions or concerns about this recall should contact Medtronic Customer Service at 800-854-3570.


 March 31, 2025
March 31, 2025