April 27, 2017 — Mercator MedSystems Inc. announced the first patient enrollment into the TANGO (Temsirolimus Adventitial Delivery to Improve Angiographic Outcomes Below the Knee) clinical trial. TANGO will study the effects of using Mercator’s proprietary Bullfrog Micro-Infusion Device for the adventitial delivery of the drug Torisel (temsirolimus) after revascularization of lesions below the knee (BTK) in patients with critical limb ischemia (CLI).
TANGO is the fourth clinical trial ongoing with the Bullfrog device in BTK (the third in the U.S.), further broadening Mercator’s portfolio of targeted drug delivery for peripheral artery disease. While other trials with the Bullfrog device are examining anti-inflammation or reduction of elastic recoil, temsirolimus reduces cellular proliferation to limit restenosis. Outside of trials using the Bullfrog device for drug delivery, only one intravascular drug delivery technology (a paclitaxel-coated angioplasty balloon) is being investigated in a single U.S. BTK study.
“This is the first clinical trial of its kind. While studies have been done outside the U.S. with -limus-eluting stents in focal lesions in the legs, the local delivery of a -limus agent without a permanent implant and in lesions longer than 5 centimeters (2 inches) has not yet been studied in the U.S.,” said Ian Cawich, M.D., principal investigator for TANGO and an interventional cardiologist at the Arkansas Heart Hospital. “We are thrilled to be a part of the TANGO study and to be at the forefront of utilizing this novel approach to address the tremendous medical need for an effective CLI treatment.”
TANGO is a Phase 2, prospective, multi-center, randomized, dose-escalation trial recruiting approximately 60 patients who have CLI related to arterial obstructions in BTK arteries. In these patients, Torisel will be delivered into the tissue immediately around the arterial wall after balloon angioplasty or mechanical atherectomy is used to open the obstructions. Torisel, a Pfizer drug, belongs to a family of well-known agents (the -limus analogs) that are commonly delivered by coronary drug-eluting stents following revascularization of lesions. The use of Mercator’s Bullfrog delivery system in the TANGO study allows the local delivery of this agent in BTK lesions, but eliminates the need to leave behind an implant in the body. While the small and tortuous anatomy of the BTK vessels can make navigation and drug delivery from a coated balloon difficult, the small profile of the Bullfrog device allows it to adjust well to small vessels and it is able to deliver drug volumes that are independent of vessel size.
The TANGO study was approved by the U.S. Food and Drug Administration (FDA) under an Investigational New Drug (IND) application. The trial is being funded by The National Heart, Lung and Blood Institute (NHLBI), a part of the National Institutes of Health (NIH) under Award Number R44HL102998.
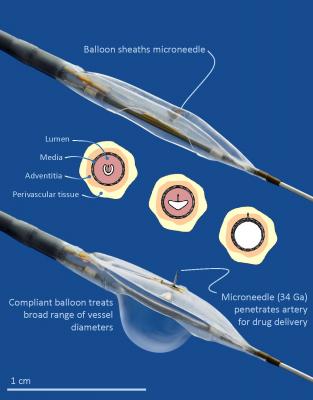
The Mercator Bullfrog Micro-Infusion Device is a U.S. Food and Drug Administration (FDA) 510(k)-cleared and CE-Marked system that infuses therapeutic and diagnostic agents directly, non-systemically and safely through blood vessel walls into adventitial tissues. The closed balloon provides a protective covering for a tiny, perpendicular-oriented injection microneedle as it is guided safely through the vascular system to target vessels with diameters of 2 to 8 millimeters.
For more information: www.mercatormed.com


 November 08, 2024
November 08, 2024 









