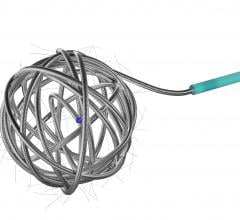
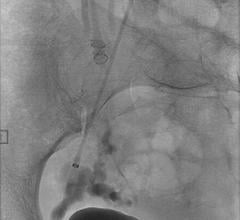
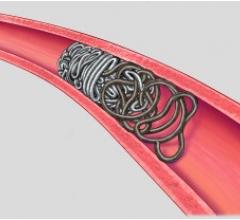
July 11, 2008 - Micrus Endovascular Corp. said this week it received approval from the Ministry of Health, Labour and Welfare (MHLW) to market in Japan the Cerecyte microcoil product line, including the MicruSphere, Presidio, HeliPaq and UltiPaq Cerecyte embolic coils, for the endovascular treatment of cerebral aneurysms.
Sales of the company’s Cerecyte microcoils will be managed by Goodman Co., Ltd., Micrus Endovascular’s exclusive distributor in the Japanese market.
Micrus develops, manufactures and markets implantable and disposable medical devices for use in the treatment of cerebral vascular diseases. Micrus products are used by interventional neuroradiologists, interventional neurologists and neurosurgeons to treat both cerebral aneurysms responsible for hemorrhagic stroke and intracranial atherosclerosis, which may lead to ischemic stroke.
For more information: www.micruscorp.com


 June 05, 2025
June 05, 2025