November 20, 2013 — Neovasc Inc. reported the results from its COSIRA (coronary sinus reducer for treatment of refractory angina)
trial assessing the efficacy and safety of the Neovasc Reducer, a percutaneous device for the treatment of refractory angina. The data shows that the Reducer achieved its primary endpoint, significantly improving the symptoms and functioning of patients disabled by previously untreatable refractory angina. The COSIRA trial also confirmed that the Reducer is safe and well tolerated, with no reports of device-related serious adverse events. The safety and efficacy data from the randomized, controlled COSIRA trial is consistent with results seen in previous non-randomized pilot studies of the Reducer.
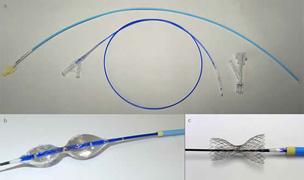
The Reducer is CE-marked in the European Union for the treatment of refractory angina. The Reducer provides relief of angina symptoms by altering blood flow in the heart's venous system, thereby increasing the perfusion of oxygenated blood to ischemic areas of the
heart muscle. Placement of the Reducer is performed using a minimally invasive transvenous procedure that is similar to implanting a coronary stent and takes approximately 20 minutes.
The COSIRA trial is a prospective, multicenter, sham-controlled, randomized, double-blinded study assessing the safety and efficacy of the Reducer in 104 patients in the European Union and Canada. Patients were randomized one-to-one between treatment and sham control arms. Its primary endpoint is a two-class improvement six months after implantation in patients' ratings on the Canadian Cardiovascular Society (CCS) angina grading scale, a four-class functional classification that is widely used to characterize the severity of angina symptoms and disability. Only patients with severe angina, CCS Class 3 or 4, were enrolled in the COSIRA trial.
The complete results of the COSIRA trial are being submitted as a Late-Breaking Clinical Trial presentation at ACC. 14, the 63rd Annual Scientific Session & Expo of the American College of Cardiology that will take place March 29-31, 2014 in Washington, D.C.
For more information: www.neovasc.com